|
|
 5 nm Nanogold® Labeling Reagents: the power of Nanogold® in a 5 nm Size 5 nm Nanogold® Labeling Reagents: the power of Nanogold® in a 5 nm Size
Updated: May 8, 2025
|
On this page:
Product information and resources
|
|
|
|
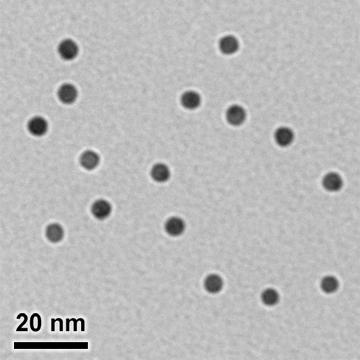
5 nm Nanogold® brings the power and versatility of Nanogold® in a larger, more readily visualized 5 nm size. Like our smaller 1.4 nm Nanogold® reagents, it covalently reacts at specific sites under mild buffer conditions. This gives a well defined product that can be purified chromatographically or by ammonium sulfate precipitation. Unlike conventional colloidal gold, 5 nm Nanogold® does not require any additional macromolecules for stabilization. Conjugate probes are smaller than colloidal gold probes and achieve higher resolution, more precise and more nearly quantitative labeling.
Unconjugated 5 nm Nanogold®, showing monodisperse, highly regular, spherically symmetrical 5 nm gold core and uniform, thin and consistent ligand coating. This ensures a highly regular particle size, smaller probe size, and reliable, reproducible labeling and EM results.
|
Make any molecule a gold probe
Nanogold® brings the versatility of fluorescent conjugation to gold labeling. Now you have a choice of sizes for Nanogold®: 1.4, 5 or 12 nm, enabling multiple labeling for electron microscopy without silver or gold enhancement!
- Antibodies
- Proteins
- Peptides
- Oligonucleotides and nucleosides: DNA, RNA and PNA
|
- Nanostructured materials
- Hormones
- Polymers
- Surfaces
|
Features and Advantages
- Stable, soluble and biocompatible: Designer coating gives solubility and biocompatibility for in vivo use. No residual charge means minimal non-specific binding.
- Highly monodisperse. SIze variation less than 15% for 5 nm gold. 1.4, 5 and 12 nm Nanogold® enable up to triple labeling with non-overlapping sizes
- Molecular precision: make smaller primary probes that get the gold closer to the target.
- Site-specific conjugation enables programmed incorporation into nanostructured materials or components of self-assembling systems.
- No need for additional proteins or polymers: smaller probes penetrate better and label more densely.
- Easily enhanced. Silver or gold enhancement enlarges while retaining monodispersity - or affords super-sensitive detection for blots and optical systems.
- Pick the optimum size: smaller for high resolution, molecular labeling, larger for visualization on a cellular scale.
Although larger than our 1.4 nm Nanogold®, our new 5 nm Nanogold® is still relatively small in size, and highly uniform. Because it does not require additional proteins for stabilization, 5 nm Nanogold® conjugates are smaller than most conjugates prepared with conventional colloidal gold and uniform in overall size, giving them faster and more consistent penetration into specimens.
If larger particle sizes are needed, 5 nm Nanogold® may easily be enhanced with GoldEnhance™ gold enhancement reagents, or our HQ Silver™ or LI Silver™ silver enhancement reagents: Depending on the reagents used, a brief exposure (1 - 4 minutes) produce highly visible grains 10-20 nm in size for electron microscope identification, while longer development times produce black signals, readily differentiated from organic chromogens, that are easily seen in the light microscope and on immunoblots, gels and Western blots.
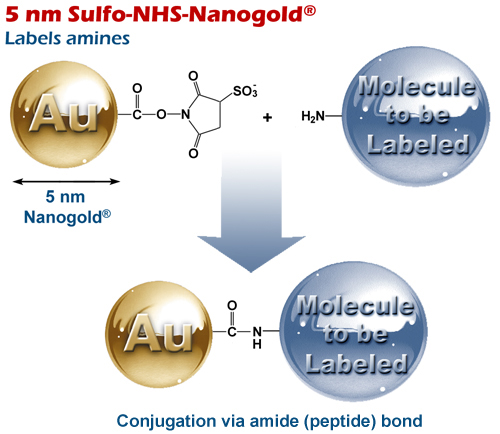
 5 nm Sulfo-NHS-Nanogold® 5 nm Sulfo-NHS-Nanogold®
|
Schematic showing reaction of 5 nm Sulfo-NHS-Nanogold® with an amino-functionalized molecule.
5 nm Sulfo-NHS-Nanogold® is a highly monodisperse, highly water-soluble, stable, and biocompatible 5 nm gold nanoparticle, with a hydrophilic coating, modified with Sulfo-NHS groups that react with aliphatic amines under mild conditions (pH 7.5 to 8.2).
Use our unique Nanogold® labeling reagents just as you would use fluorescent labeling reagents. Labeling with 5 nm Sulfo-NHS-Nanogold®, like the smaller 1.4 nm Sulfo-NHS-Nanogold®, is straightforward: Simply mix reconstituted 5 nm Sulfo-NHS-Nanogold® with the target molecule for one hour at room temperature or 16 h in the refrigerator.
Once reaction is complete, conjugates may be chromatographically separated by gel filtration. Alternatively, labeled proteins may be isolated by ammonium sulfate precipitation, and oligonucleotides by ethanol precipitation or gel electrophoresis. Ion exchange chromatography or hydrophobic interaction chromatography may also be useful. The sulfo-NHS group specifically reacts with an amine under mild conditions to produce a stable, covalent amide bond.
|
Selectively label any molecule with an accessible amino- group
- Antibody IgG and IgM: label at an N-terminal amine or lysine residue - no need for reduction or other pretreatment. Conserve rare or low abundance antibodies.
- Antibody Fab or ScFv fragments: label small antibody fragments without unique thiol sites.
- Proteins and peptides: Label an N-terminal amines or lysine residues. Disulfide bridges remain intact: preserve tertiary and quaternary structures.
- DNA, RNA and PNA: Use an amino-modified phosphoramidite or lysine during synthesis to put an amine right where you want the gold. Amino- modifications may be introduced anywhere - you are not restricted to 3' or 5' terminal labeling.
- Nanostructured materials: label an amino-functionalized building block, then assemble to make programmed gold nanoparticle arrays. Or program amino- labeling sites into self-assembled materials and add the gold afterwards.
- Small molecules: Decorate 5 nm Nanogold® with small molecules such as hormones, inhibitors, or signalling molecules, then localize binding sites with molecular precision.*
* 5 nm Sulfo-NHS Nanogold® is polyfunctional, so labeling reactions need to be planned to avoid cross-linking and control the ratio of Nanogold® to conjugate biomolecule. See the product information and instructions for more information and suggestions.
Product information
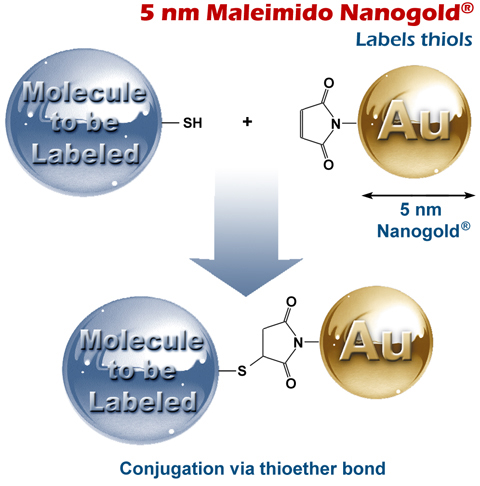
 5 nm Maleimido Nanogold® 5 nm Maleimido Nanogold®
|
Schematic showing reaction of 5 nm Maleimido Nanogold® with a thiol-functionalized molecule.
5 nm Monomaleimido Nanogold® is a highly monodisperse, highly water-soluble, stable, and biocompatible 5 nm gold nanoparticle, with a hydrophilic coating, modified with maleimide groups that react with thiols (sulfhydryls) under mild conditions (pH 6.0 to 7.0).
Use our unique Nanogold® labeling reagents just as you would use fluorescent labeling reagents. Labeling with 5 nm Monomaleimido Nanogold®, like the smaller 1.4 nm Monomaleimido Nanogold®, is straightforward: Simply mix reconstituted 5 nm Monomaleimido Nanogold® with the target molecule for one hour at room temperature or 16 h in the refrigerator.
Once reaction is complete, conjugates may be chromatographically separated by gel filtration. Alternatively, labeled proteins may be isolated by ammonium sulfate precipitation, and oligonucleotides by ethanol precipitation or gel electrophoresis. Ion exchange chromatography or hydrophobic interaction chromatography may also be useful. The maleimide group specifically reacts with a thiol under mild conditions to produce a stable, covalent thioether bond.
|
|
Selectively label any molecule with an accessible thiol group:
- Antibody IgG and IgM: label at a hinge thiol, remote from the binding site - preserve affinity.
- Antibody Fab' fragments: attach the 5 nm Nanogold® at the hinge thiol at the opposite end to the binding site - preserve maximum native affinity.
- Proteins and peptides: Label selectively at a unique thiol site, or incorporate a Cys residue during peptide synthesis at the site you wish to localize.
- DNA, RNA and PNA: Use a thiol-modified phosphoramidite or cysteine during synthesis to put a thiol right where you want the gold - or modify enzymatically in situ to place the gold at the modification site.
- Nanostructured materials: label a building block, then assemble for a programmed gold nanoparticle array, or program specific labeling sites into a nanostructure and add the gold afterwards.
- Small molecules: Decorate 5 nm Nanogold® with small molecules such as hormones, inhibitors, or signalling molecules, then localize binding sites with molecular precision.*
* 5 nm Maleimido Nanogold® is polyfunctional, so labeling reactions need to be planned to avoid cross-linking and control the ratio of Nanogold® to conjugate biomolecule. See the product information and instructions for more information and suggestions.
Product information
 5 nm Azido (Click) Nanogold® 5 nm Azido (Click) Nanogold®
5 nm Click Nanogold® reagents are available with three different reactivities to let you label alkyne-modified or azide-modified targets, using either copper-catalyzed (CuAAC) or copper-free (SPAAC) reactions.
5 nm Azido Nanogold® reacts with alkynes and related reagents; may be used for copper-catalyzed (CuAAC) or copper-free (SPAAC) click reactions.
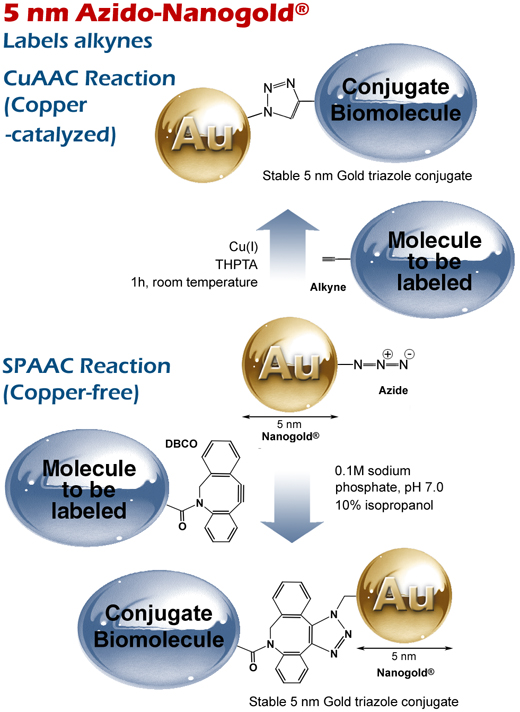
Reactions of 5 nm Azido-Nanogold® with
(top) a non-strained alkyne via copper-catalyzed (CuAAC) click reaction, and
(bottom) a strained alkyne via strain-promoted (SPAAC) copper-free click reaction.
|
Applications:
- Label dynamic processes in living cells or tissues - Click reagents will not hydrolyze or degrade.
- Label components in living cells: do not react with endogenous species like biotin or cross-reactive proteins.
- High-resolution labeling of subunits or molecular sites: just incorporate the corresponding reactive azide or alkyne into the target - no antibody or probe required.
- Optical Visualization: use silver or gold enhancement to visualize by light microscopy or for super-sensitive blotting. See as little as 1 pg of target IgG!
- Multiplex your labeling: Add a click reaction to detect an additional target if there is no suitable antibody or probe.
|
|
Product information
 5 nm Alkyne (Click) Nanogold® 5 nm Alkyne (Click) Nanogold®
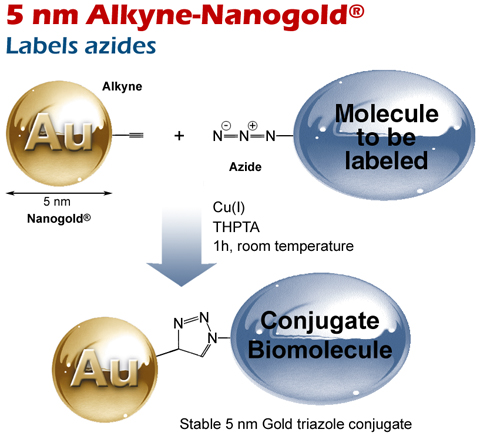
5 nm Alkyne Nanogold® reacts with azides via copper-catalyzed (CuAAC) reaction.
|
|
Reaction of 5 nm Alkyne-Nanogold® with (top) an azide via copper-catalyzed (CuAAC) click reaction.
|
Applications:
- Label dynamic processes in living cells or tissues - Click reagents will not hydrolyze or degrade.
- Label components in living cells: no reaction with endogenous species or cross-reactive proteins.
- High-resolution precision labeling - no antibody or probe.
- Optical Visualization: use silver or gold enhancement to visualize by LM or for super-sensitive blotting.
- Multiplex your labeling: Use click reactions when no antibody or probe is available.
|
Product information
 5 nm DBCO (Click) Nanogold® reacts with azides via copper-free SPAAC reaction. 5 nm DBCO (Click) Nanogold® reacts with azides via copper-free SPAAC reaction.
5 nm Click Nanogold® reagents are available with three different reactivities to let you label alkyne-modified or azide-modified targets, using either copper-catalyzed (CuAAC) or copper-free (SPAAC) reactions.
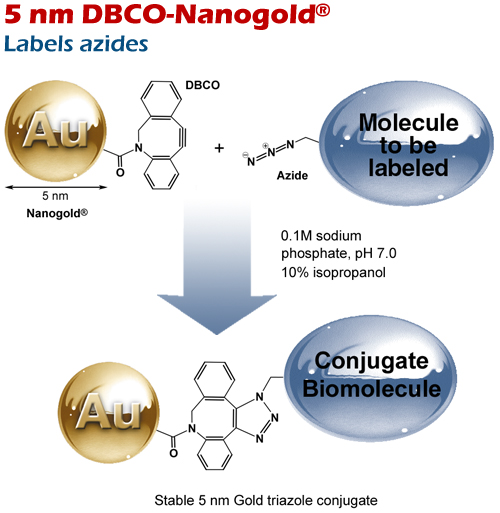
5 nm DBCO Nanogold® reacts with azides via copper-free SPAAC reaction.
|
Reaction of 5 nm DBCO-Nanogold® with a strained alkyne (cyclooctyne derivative) via strain-promoted (SPAAC) click reaction.
|
Applications:
- Label dynamic processes in living cells or tissues - Click reagents will not hydrolyze or degrade.
- Label components in living cells: no reaction with endogenous species or cross-reactive proteins.
- High-resolution precision labeling - no antibody or probe.
- Optical Visualization: use silver or gold enhancement to visualize by LM or for super-sensitive blotting.
- Multiplex your labeling: Use click reactions when no antibody or probe is available.
|
|
Product information
 5 nm Amino Nanogold® 5 nm Amino Nanogold®
Selectively label unique sugar or activated carboxyl sites
Positively ionizing amino- groups enable charge-based staining or labeling of negatively charged targets
- Carboxyl labeling: Label at carboxylic acid residues by converting the carboxylic acid to an activated form for reaction with 5 nm Amino Nanogold®.
- Carbohydrate labeling: use periodate or other mild, selective oxidants to oxidize carbohydrate sugars to aldehydes, then react with 5 nm Amino Nanogold® and reduce the resulting imides to give stable, amine-linked conjugates. See our Application note on RNA labeling for details.
- Protein labeling: activate 5 nm Amino Nanogold® using amine-reactive cross-linkers cross-link to a variety of other functional groupsor targets.
- Charge-based labeling: Under slightly acidic conditions, 5 nm Amino Nanogold® protonates and assumes a positive charge: use to label negatively ionizing regions in proteins or organelles.
- Make DNA nanowires: like the smaller 1.4 nm Positively Charged Nanogold®, 5 nm Amino Nanogold® can bind to the negatively charged groups in the DNA backbone; see our Microscopy & Microanlaysis 2001 paper for details. silver or gold enhance to make conductive nanowires.
* 5 nm Amino Nanogold® is polyfunctional, so labeling reactions need to be planned to avoid cross-linking and control the ratio of Nanogold® to conjugate biomolecule. See the product information and instructions for more information and suggestions.
5 nm Amino Nanogold® is a highly monodisperse, highly water-soluble, stable, and biocompatible 5 nm gold nanoparticle, with a hydrophilic coating, modified with Sulfo-NHS groups that react with aliphatic amines under mild conditions (pH 7.5 to 8.2).
Use our unique Nanogold® labeling reagents just as you would use fluorescent labeling reagents. Labeling with 5 nm Amino-Nanogold®, like the smaller 1.4 nm Amino-Nanogold®, is straightforward: reconstitute the 5 nm Amino Nanogold®, activate with the appropriate cross-linker, then incubate with the target molecule under suitable conditions.
Once reaction is complete, conjugates may be chromatographically separated by gel filtration. Alternatively, labeled proteins may be isolated by ammonium sulfate precipitation, and oligonucleotides by ethanol precipitation or gel electrophoresis. Ion exchange chromatography or hydrophobic interaction chromatography may also be useful. The maleimide group specifically reacts with a thiol under mild conditions to produce a stable, covalent thioether bond.
Product information
 5 nm Non-Functional Nanogold® - Instructions 5 nm Non-Functional Nanogold® - Instructions
5 nm Non-Functional Nanogold® is a highly soluble, non-ionizing, regular 5 nm coated gold nanoparticle.
- Use as a fiducial marker: 5 nm Non-Functional Nanogold® is highly regular, stable and biocompatible. Use as a small fiducial marker for aligning high-resolution tilt sections or for electron tomography.
- Use for sizing: 5 nm Non-Functional Nanogold® is highly regular in size: the gold nanoparticle is 5 nm in diameter, and the overall diameter, including the shell, is close to 10 nm. Use to determine size limits for crossing cellular junctions or for sizing pores.
- Use for mapping: 5 nm Non-Functional Nanogold® is highly water-soluble, stable, and non-ionizing. It has minimal affinity for proteins or cellular components, and therefore may be used to map contiguous internal spaces, such as neurons or vacuoles, or connectivity.
.
 Custom 5 nm Nanogold® Conjugates and Labeling Custom 5 nm Nanogold® Conjugates and Labeling
Nanoprobes can perform custom conjugation using any of our Nanogold®, undecagold or colloidal gold labels with primary antibodies, proteins, peptides, DNA, RNA or PNA, small molecules, nanomaterials components, surfaces or other materials. Custom FluoroNanogold™ labeling is available for antibodies or larger proteins. This includes the purification and characterization of conjugates. Call 877-447-6266 (US toll-free number) or (631) 205-9490, use our custom synthesis quotation form, or e-mail us at tech@nanoprobes.com to discuss your project and obtain a quote.
|
|







