|
|
Got a question? We can help.
Talk with our scientists in Tech Support.
We're scientists too, and we use all our products in our own research.
We look forward to helping you with your project!
FAQ
See also
- Guide to Gold Nanoparticle Labeling
Detailed description of gold nanoparticle labeling, tips and tricks for successful conjugations, isolation of conjugates, and how to calculate labeling.
I don't want to use all the reagent at once. Can I dissolve it
and keep some for later?
Maleimido- and Sulfo-NHS-Nanogold®: The maleimido- and sulfo-NHS groups are hydrolyzed in hours in aqueous solution. In order to prevent this, they must be dissolved in rigorously dry DMSO or dimethylacetamide (DMA); provided all traces of moisture are kept out of these solutions it may be
possible to preserve the reactivity, but we cannot guarantee it. If you have multiple experiments, you can order Nanogold® and undecagold labeling reagents in five smaller aliquots instead of one vial; see catalog information.
I am planning to label a molecule, such as a peptide or a small molecule, which has not been labeled with Nanogold® before, so there is no reported procedure for separating the labeled product from the reaction mixture. Usually, this molecule is separated by gel electrophoresis, ion exchange or reverse-phase chromatography. Can I use these techniques to separate Nanogold® conjugates?
We recommend gel filtration as the method of choice for separating Nanogold® conjugates: this is routinely used in our laboratories, and the method is wel characterized. Nanogold® has a molecular weight close to 15,000, but because a substantial fraction of this is made up of very dense gold atoms, in many separation techniques that are based on size, such as gel filtration, Nanogold behaves as though its MW were closer to about 8,000.
When planning your labeling reaction, you should use an excess of the component which is more easily separated from the Nanogold®-labeled product on the basis of size. If your molecule is smaller than Nanogold®, you should use an excess of the smaller molecule for your labeling reaction (about a five-fold excess would be a good starting point for slightly smaller molecules, 10-fold to 20-fold for much smaller molecules), because it is easier to separate excess smaller molecules from the reaction mixture than excess larger Nanogold®.
If you are labeling a molecule larger than about 10,000 MW, you should use an excess of Nanogold®, since this is now more readily separated from the conjugate than excess unreacted biomolecule. A two-fold to five-fold excess is appropriate for most reactions.
A list of gel filtration media, with their separation characteristics, is given in our Guide to Conjugate Isolation.
Other chromatographic methods have been tried, but have not so far given reproducible results. One approach which was moderately successful for undecagold, and appears promising for Nanogold®, is hydrophobic interaction chromatography. The advantage of this method is that it can be used to separate both larger and smaller impurities from the Nanogold conjugate; pocedure is based on the separation of undecagold, undecagold-Fab' conjugates, and unlabeled Fab' described by Hainfeld:
Hainfeld, J. F. Undecagold-antibody method. In Colloidal Gold: Principles Methods, and Applications., M.A. Hayat (Ed.), San Diego, Academic Press; Vol. 1>, pp. 413-429 (1989).
It should be noted that the technique used is hydrophobic interaction chromatography, not reverse-phase; the method relies on a gradient of decreasing ionic strength, in which the different immobilized molecules eluet at different ionic strengths. For both the undecagold and Nanogold experiments, a butyl-derivatized gel was used; a biphenyl derivatized gel was also tried, but the butyl gel gave the best results.
A gradient of three to six column volumes was used for the separation. The conjugate was dissolved in 2.0 M ammonium sulfate, with pH adjusted to a neutral value (7 or 7.5) using triethylammonium acetate (5 mM). The two buffers used for the gradient were:
(A) 2 M ammonium sulfate, with the pH adjusted to a neutral value (6.5, 7 or 7.5) using triethylammonium acetate (5 mM).
(B) 5 mM triethylammonium acetate, pH 7 or 7.5.
After sample injection, one volume of buffer A was run, then over the gradient, the proportion of buffer B was steadily increased from 0 to 100 %, then elution was continued with buffer B. Using this gradient, unconjugated Fab' was eluted first, followed by Nanogold-Fab', followed by unattached Nanogold.
We do not recommend gel electrophoresis as a purification method, because the migration of the Nanogold® particle through gels can be unpredictable and the identification of conjugates is not straightforward. Dialysis has given mixed results; however, caution shoud be exercised since some membranes contain components that can dgrade Nanogold®.
How can I label nucleotides with Nanogold?
Nanoprobes has an ongoing research interest in preparing gold labeling reagents for labeling DNA, RNA and other nucleic acids. Monoamino Nanogold®, Monomaleimido Nanogold® and Mono-sulfo-NHS-Nanogold® can all be used to label nucleotides. Specific reactivity towards the Nanogold® reagent must first be incorporated into the nucleotide at the desired labeling site. This can be achieved in three ways:
5'-Labeling with Monoamino Nanogold® (For all oligonucleotides):
First, de-phosphorylate the 5' terminus enzymatically. Convert the residual phosphate group to a reactive ester using either CDI (N,N'-carbonyldiimidazole), or EDC (1-ethyl-3,3'-dimethylaminopropyl carbodiimide) with sulfo-NHS or imidazole. React the activated ester with Monoamino-Nanogold®; it will be conjugated via an amide linkage, as shown in scheme 1:
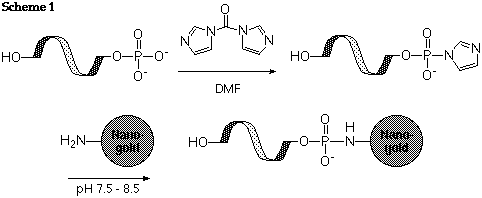
Reference:
- Chu, B. C. F.; Kramer, F. R., and Orgel, L. E.; Nucleic Acids Res., 1986, 14, 5591.
Thiolation of 5' terminus and Labeling with Monomaleimido-Nanogold® (For all oligonucleotides):
De-phosphorylate and activate with CDI or EDC as described above. React with 2,2'-dithiobis (ethylamine) dihydrochloride (cystamine dihydrochloride) as described by Chu (Ref. 1), or alternatively treat a 5'-phosphorylated nucleotide with imidazole, cystamine and EDC (Ref.2). Reduce with 0.1 M DTT and separate rigorously from excess DTT to generate the free thiol as described by Ghosh (Ref. 2). Label the resulting thiolated oligonucleotide with Monomaleimido Nanogold® as shown in scheme 2:
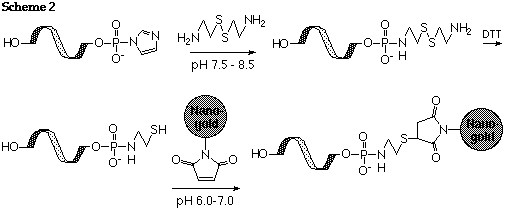
References:
- Chu, B. C. F.; Kramer, F. R., and Orgel, L. E.; Nucleic Acids Res., 1986, 14, 5591.
- Ghosh, S. K.; Kao, P. M.; McCue, A. W., and Chappelle, H. L.; Bioconjugate Chem., 1990, 1, 71.
Labeling at reactive group introduced via substituted phosphoramidite (For synthetic oligonucleotides):
Use a commercially available modified phosphoramidite reagent to introduce a protected amine or a protected thiol at a specific base (These reagents are available from Clontech or Glen Research). Then deprotect according to the recommended protocol for the substituted phosphoramidite, and label with Monomaleimido Nanogold® or Mono-sulfo-NHS-Nanogold®.
References:
- Alivisatos, A. P., Johnsson, K. P., Peng, X., Wilson, T. E., Loweth, C. J., Bruchez, M. P., Jr., and Schultz, P. G.: Organization of 'Nanocrystal Molecules' using DNA. Nature, 382, 609 (1996).
If these approaches are not applicable to your system, you can use Positively Charged Nanogold®. This reagent is functionalized with multiple amines, and adopts a positive charge at neutral or acidic pH. It will then bind electrostatically to the negatively charged phosphate groups of oligonucleotides.
Alternatively, introduce a hapten such as biotin into your oligonucleotide probe, either by biotinylation of the nucleotide or by incorporation of a biotin-modified phosphoramidite during synthesis. Then detect the biotinylated nucleotide using Nanogold® anti-biotin (IgG or Fab') or Streptavidin. We hope to introduce gold-labeled anti-digoxigenin as soon as one of the antibody suppliers begins to carry the unlabeled antibody at a more affordable price.
What volume should I do the reaction in?
As small as possible. It is best if you can dissolve everything so that the concentration of Nanogold® is 10 nmol/mL or greater, but 5 nmol/mL should still give good labeling.
The reagent isn't dissolving as well as it should. What can I do?
Tha Nanogold® reagent should be dissolved in a small volume of organic solvent, then diluted to the final volume with water. The best organic solvent is DMSO; however, isopropanol or DMA (N,N-dimethylacetamide) are also good solvents. Vortexing will also help speed solution.
Note: Monomaleimido and Mono-Sulfo-NHS-Nanogold® are supplied lyophilized from buffered solution. The buffer will not dissolve in the organic solvent, so the sample may still appear granular. If the solid is colorless, it is buffer salt - and it will dissolve when you add water or aqueous buffer to the reagent.
I have high levels of background staining. What can I do to lower them?
There are two approaches to reducing background staining: (a) modify the experimental conditions before and during silver enhancement; or (b) improve the "stopping" of the silver enhancement reaction or apply back development after it is complete.
Reducing background during reaction
In experiments with the combined fluorescein and Nanogold® probe FluoroNanogold, it was found that washing with 0.02 M sodium citrate buffer, pH 7.0, before silver enhancement gives significantly lower backgrounds than many other treatments. This was found to be effective when the Danscher silver enhancer was used for development. When Nanoprobes HQ Silver was used, 0.02 M sodium citrate buffer at pH 3.5 immediately before silver enhancement gave lower background. In immunoblots, we find that 0.05 M disodium EDTA, pH 4.6, before silver enhancement also helps minimize background.
Reference:
- Powell, R. D.; Halsey, Carol M. R.; Spector, D. L.; Kaurin, S. L.; McCann, J.;, and Hainfeld, J. F. A covalent fluorescent-gold immunoprobe: "simultaneous" detection of a pre-mRNA splicing factor by light and electron microscopy. J. Histochem. Cytochem., 45, 947-956 (1997).
Addition of a small amount of the detergent Tween-20 to the Nanogold® incubation buffer helps to prevent hydrophobic interactions, which can be another source of non-specific binding. If the sample can tolerate it, washing with 0.6 M triethylammonium bicarbonate buffer, in which Nanogold® is highly soluble, can also lower background. Preparation of triethylammonium bicarbonate buffer is described in the following reference:
- Safer, D.; Bolinger, L., and Leigh, J. S.; J. Inorg. Biochem., 26, 77 (1986).
"Stop" Reactions and back development:
Under most circumstances, repeated washing with deionized or distilled water will be sufficient to halt the silver enhancement process. However, it may continue within the specimen, and this can sometimes give over-development or a dark appearance in the light microscope. In this case, one of the following methods may be used to "stop" the silver enhancement reaction:
- 1% acetic acid.
- 1% acetic acid followed by photographic fixer (Agefix, Agfa-Gevaert, or Ilfospeed 200, Ilford).
- direct photo fix, using those just mentioned.
- brief rinse in 2.5% sodium chloride.
- 15-25% aqueous sodium thiosulfate plus 15% sodium sulfite.
- 1% acetic acid, washes in acetate buffer, toning in 0.05% HAuCl4 3-10 min, with excess silver removed with 3% sodium thiosulfate. We found that Nanogold-labeled proteins run on a polyacrylamide gel kept low backgrounds when stopped with 10% acetic acid with 10% glucose in water, as opposed to just a water stop.
These methods, and their references, are discussed in our recent review of Nanogold® technology:
In the event that the reaction has proceeded too far, it may be "back-developed" to remove the excess background staining by treatment with Farmer's solution (0.3 ml 7.5% potassium ferricyanide, 1.2 ml of 20% sodium thiosulfate, 60 ml water). Application of this solution briefly to your sample before gold toning may help to remove backgroud silver deposition.
Reference:
- Danscher, G.: Histochemistry, 71, 1-16 (1981).
Any tips for other users?
Things you'd like to see us make?
Tell us about your question at tech@nanoprobes.com.
|