Updated: July 13, 2004
N A N O P R O B E S E - N E W S
Vol. 5, No. 7 July 13, 2004
In this Issue:
|
This monthly newsletter is to inform you about techniques to improve your immunogold labeling, highlight interesting articles and novel applications of metal nanoparticles, and answer your questions. We hope you enjoy it and find it useful; as always, let us know if we can improve anything.
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Make your own new Nanogold® labeling reagents
Although we offer Nanogold® in a range of functionalities that addresses most common labeling needs, you have many other options available. For example, you may wish to label ketones, or introduce a chemically or photolytically cleavable cross-link into your conjugate. Monoamino Nanogold contains close to one primary aliphatic amine. This may be converted to a variety of reactive functional groups using heterobifunctional cross-linkers available from companies such as Pierce or Molecular Biosciences to prepare Nanogold reagents with novel reactivities.
|
You can conduct this type of activation reaction quite simply, using a heterobifunctional cross-linker that has N-hydroxysuccinimide (NHS) or Sulfo-NHS at one end and the desired reactivity at the other. React the Monoamino Nanogold with an excess of the heterobifunctional cross-linking reagent, to drive reaction of the Nanogold to completion (usually a 20-fold excess is a good starting point) under conditions that allow the NHS end of the cross-linker to react with the Monoamino Nanogold, most commonly HEPES, phosphate or phosphate-buffered saline (PBS) at pH 7.5 to 8.2; then separate the activated gold from excess cross-linker by gel filtration over a desalting gel such as GH25 (available from Millipore) or a similar material.
Polyfunctional Nanogold®
Monomaleimido Nanogold, Mono-Sulfo-NHS-Nanogold, and Monoamino Nanogold, as their names imply, are tailored for conjugation to a single biomolecule. But you want to make more than one cross-link. How?
We do offer two reagents, Positively charged Nanogold and Negatively Charged Nanogold that offer multiple functionalities. Positively Charged Nanogold contains an estimated three to six primary amines, which may be activated with heterobifunctional cross-linking reagents as described above; and Negatively Charged Nanogold contains several carboxylic acid groups. These may be activated using 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide Hydrochloride (EDC) with Sulfo-NHS to form activated esters that react rapidly and selectively with amines.
Reference:
Staros, J. V.; Wright, R. W., and Swingle, D. M.: Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions; Anal. Biochem., 156, 220-222 (1986).
Once you have introduced multiple reactive groups, the stoichiometry of your labeling reaction may be controlled by two factors: the space available around the Nanogold, and the ratio of your reagents. Whether space is a factor depends on the size of your biological molecule. If you are using a protein or large peptide, for example, you may only be able to link two or three biomolecules before steric hindrance prevents any more from approaching; with a smaller molecule, more copies may be linked. If you wish to limit labeling to a smaller number of biomolecules per gold particle, use a ratio of biomolecule to activated gold that ensures that this is the majority species. For example, a 2 : 1 mixture of biomolecule : Nanogold should ensure that the majority product contains two biomolecules per Nanogold.
If you'd like our help in planning one of these less typical conjugation reactions, please contact our technical support people. We will be glad to advise.
More information:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
Kowa and co-workers describe double immunogold labeling to localize two component proteins in amyloid plaques in the latest issue of the American Journal of Pathology, using silver-enhanced Nanogold® and 10nm colloidal gold. This group are using a number of novel monoclonal antibodies to characterize the chronological and spatial relationship of Collagenous Alzheimer amyloid plaque component (CLAC), a unique non-Abeta amyloid component of senile plaques (SP) derived from a transmembrane collagen termed CLAC-precursor, with other components of SP amyloid in the brains of patients with Alzheimers disease (AD), Down syndrome (DS), and of PSAPP transgenic mice. Double immunogold labeling was used to compare the ultrastructural distribution of CLAC with that of the corresponding Abeta amyloid components.
Double immunolabeling for electron microscopic observation of CLAC/Abeta42 or CLAC/Abeta40 in AD cortices was performed on 50-micrometer thick floating sections fixed in 10% formalin for 24 hours. First, these were incubated with monoclonal antibody 9D2 overnight. After washing, they were incubated with Nanogold-labeled anti-mouse IgG antibody for 24 hours; after washing in 10 mM phosphate buffer, sections were post-fixed in 2.5% glutaraldehyde (1 hour), transferred to 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES) buffer (pH 5.8), washed in distilled water (DW), and enhanced using HQ Silver. After stopping silver enhancement by washing in DW, the sections were post-fixed in 2% osmium tetroxide for 1 hour, dehydrated, and embedded in epoxy resin. Next, post-embedding immunolabeling for Abeta40/42 was performed: ultra-thin sections were cut at 80 nm and treated with 3% H2O2 (10 minutes) followed by 1% sodium periodate (10 minutes) on a nickel grid. Sections were blocked in 3% bovine serum albumin in 100 mM phosphate buffer (pH 7.4) for 30 minutes, incubated with BC05 or BA27 antibodies, then reacted with 10 nm gold-labeled anti-mouse IgG. The sections were then double-stained by uranyl acetate and lead-citrate, and
viewed in electron microscope.
In AD and DS cerebral cortex, conventional immunohistochemistry and double and triple immunofluorescence showed that CLAC is colocalized with Abeta42 but often lacks Abeta40- or thioflavin S (thioS)- reactivities. Immunoelectron microscopy of CLAC-positive senile plaques showed labeling of fibrils that are more loosely dispersed, thinner and less electron-dense than typical amyloid fibrils in CLAC-negative SP. In DS cerebral cortex, diffuse plaques in young patients were negative for CLAC, whereas a subset of SP became CLAC-positive in patients aged 35 to 50 years, before the appearance of Abeta40. In DS cases over 50 years of age, Abeta 40-positive SP dramatically increased, whereas CLAC burden remained at a constant level. In PSAPP transgenic mice, CLAC was positive in the diffuse Abeta deposits surrounding huge-cored plaques. Thus, CLAC and Abeta40 or thioS exhibit mostly separate distribution patterns; this suggests that CLAC is a relatively early component of plaques in human brains and it may have inhibitory effects against the maturation of SP into beta-sheet-rich amyloid deposits.
Reference:
Kowa, H.; Sakakura, T.; Matsuura, Y.; Wakabayashi, T.; Mann, D. M.; Duff, K.; Tsuji, S.; Hashimoto, T.; and Iwatsubo, T.: Mostly Separate Distributions of CLAC- versus Abeta40- or Thioflavin S-Reactivities in Senile Plaques Reveal Two Distinct Subpopulations of beta-Amyloid Deposits. Am. J. Pathol., 165, 273-281 (2004).
For a more detailed description of the post-embedding procedure, see:
Yamaguchi, H.; Maat-Schieman, M. L.; van Duinen, S. G.; Prins, F. A.; Neeskens, P.; Natte, R., and Roos, R. A: Amyloid beta protein (Abeta) starts to deposit as plasma membrane-bound form in diffuse plaques of brains from hereditary cerebral hemorrhage with amyloidosis-Dutch type, Alzheimer disease, and non-demented aged subjects. J. Neuropathol. Exp. Neurol., 59, 723732 (2000).
More information:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
There has been a tremendous response to our new reagent, Nickel (II) nitriloacetic acid (NTA) Nanogold® (below), a new general-purpose gold labeling reagent which targets polyhistidine (His) tags. Ni-NTA-Nanogold may be used to localize expressed His-tagged targets by electron and light microscopy, and in blotting applications.
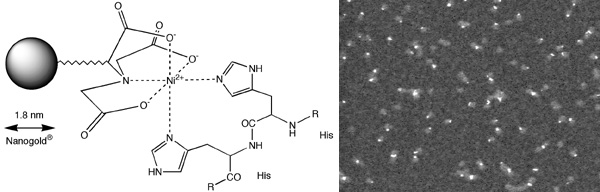
|
left: Structure of Ni-NTA-Nanogold® showing interaction with Interaction with a His-tagged protein; right: Knob protein from adenovirus cloned with 6x-His tag, labeled with Ni-NTA-Nanogold, column purified from excess gold, and viewed in the scanning transmission electron microscope (STEM) unstained (Full width approximately 245 nm).
|
Features of NTA-Ni(II)-Nanogold include:
- The nitrilotriacetic acid - Ni(II) chelate is much smaller than an antibody. It therefore has unprecedented resolution, ideal for localizing sites in proteins and protein complexes at molecular resolution. It is also better able to access restricted sites and penetrate into specimens.
- NTA-Ni(II)-Nanogold is made with a modified gold particle, stabilized slightly differently to that used for other Nanogold reagents, which results in very high solubility and stability. At 1.8 nm in size, this particle is also readily visualized by electron microscopy.
- Binding constants for Ni(II)-NTA are very high; the chelate effect of multiple histidine binding and multiple Ni(II)-NTA functionalization gives dissociation constants estimated from 10-7 to 10-13 M-1. For many applications, this provides binding strengths comparable to antibodies.
When using NTA-Ni(II)-Nanogold, you should note that the nature of the binding interaction is different from that of antibodies or other targeted biomolecules such as streptavidin. Therefore different concentrations, reagents and conditions may be appropriate for blocking and for preventing or eliminating undesirable background interactions. Binding occurs by coordination of electronegative atoms, usually aromatic nitrogen, to the nickel (II) ion; if you are labeling a target that contains other aromatic nitrogens, such as other histidine residues, these may also bind. The undesirable interaction may be eliminated by treatments that reduce this interaction. The following steps may be useful:
- Wash with a buffer containing imidazole. Imidazole is the active coordinating group in histidine; treatment with imidazole will displace isolated single histidine binding, but it will not overcome the chelate effect and stronger binding of NTA with polyhistidines, and therefore will disturb polyhistidine binding much less. Try increasing the imidazole concentration from 10 mM progressively to 200 mM until background is controlled to your satisfaction.
- Increase the ionic strength of the solution. The nitrilotriacetic acid moiety is negatively charged, and may interact with positively charged regions of a target; increasing the ionic strength will help to prevent this. Try 300 mM NaCl, and, if your biomolecule can tolerate it, increase to 1.0 M if necessary.
- Other possible factors include:
- hydrophobic interactions. These may be reduced or eliminated by the addition of detergents; try 0.05% Tween-20, and increase to 0.1% if necessary.
- pH: it may be helpful to vary the pH to find an optimum pH at which charge interactions such as those mentioned above are reduced.
- Transition metals may also promote interactions between the NTA group and electron donating groups in your specimen. Wash with 0.05 M disodium ethylene diamine tetraacetic acid (EDTA) to remove these.
- Interaction of thiols (sulfhydryls) with gold. Thiols have a strong affinity for gold, and if they are present in your specimen, any gold particle species, including Ni-NTA-Nanogold, may bind to them. Avoid the use of reducing buffers or preservatives containing thiols, such as dithiothreitol (DTT), mercaptoethanol, or mercaptoethylamine hydrochloride. If your specimen contains exposed thiols, they may be blocked with N-ethylmaleimide.
If you are seeing background signal after silver or gold enhancement, a number of methods are available for stopping these reactions and preventing further reaction after the desired end-point by reagents that have diffused into specimens.
In the development of Ni-NTA-Nanogold, it was found that a form containing multiple NTA-Ni(II) groups produced the best overall combination of labeling selectivity, density and sensitivity. However, because this can interact with polyhistidine tags on several protein molecules simultaneously, it may act to aggregate proteins, or perturb the formation of protein complexes, in solution. The best aproach to avoid this is to use a ratio of reagent to protein such that the stoichiometry reduces or eliminates this possibility. For example, if your protein has only one polyhistidine tag, then using an excess of the Ni-NTA-Nanogold reagent will guard against the possibility of multiple interactions. You can also help avoid the possibility by carefully selecting when to add the reagent, for example after complex assembly.
References:
- Hainfeld, J. F.; Liu, W.; Halsey, C. M. R.; Freimuth, P., and Powell, R. D.: Ni-NTA-Gold Clusters Target His-Tagged Proteins. J. Struct. Biol., 127, 185-198 (1999).
- Buchel, C.; Morris, E.; Orlova, E.; and Barber, J.: Localisation of the PsbH subunit in photosystem II: a new approach using labelling of His-tags with a Ni(2+)-NTA gold cluster and single particle analysis. J. Mol. Biol., 312, 371-379 (2001).
More information:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
A number of protocols have been described for double labeling using silver-enhanced Nanogold® to mark one site of interest and chromogenic enzymatic staining to mark a second for electron microscopy; the two staining patterns are readily identifiable in the microscope. This month, Wu and co-workers report another, using the two methods to detect vesicular glutamate transporter 1 (VGLUT1) and choline acetyltransferase (ChAT) immuno-reactivity respectively in the rat lumbar spinal cord as part of their study of the expression of vesicular glutamate transporters (VGLUTs: VGLUT1, VGLUT2 and VGLUT3) in muscle spindle afferents. VGLUT1 immunoreactivity was detected in the sensory endings on the equatorial and juxta-equatarial regions of intrafusal fibers as well as in many axon terminals within lamina IX of the spinal cord. In addition to being expressed on the central axon terminals, where it makes contact with ChAT-positive motoneurons, the Nanogold-silver label distribution provided evidence that VGLUT1 is also expressed in the peripheral sensory endings of muscle-spindle afferents.
Rat lumbar cord sections were placed in 20% (v/v) normal donkey serum in PBS for 1 h, then incubated at 4°C overnight with a mixture of 0.5 micrograms/mL anti-VGLUT1 rabbit IgG and 1/100-diluted anti-ChAT goat IgG in PBS containing 2% (v/v) normal donkey serum (PBS-G). Next, the sections were incubated at 4°C overnight with 1 microgram/ml of biotinylated anti-goat IgG donkey antibody in PBS-G, then placed in 10% normal goat serum for 1 h at room temperature, then incubated at 4°C overnight with 1/100-diluted Nanogold anti-rabbit IgG in PBS-G. Sections were postfixed for 10 min with 1% (v/v) glutaraldehyde in 0.1 M PB, then enhanced using (a href="../instructions/Inf2012.html">HQ Silver. The sections were then incubated at room temperature with 1/60-diluted ABC-Elite (Vector) for 4 h, and then with 0.02% (w/v) DAB and 0.001% (v/v) H2O2 in 50 mM TrisHCl (pH 7.6). The sections were washed, fixed for 40 minutes in 1% (w/v) OsO4 in 0.1 M PB, counterstained with 1% (w/v) uranyl acetate, embedded in epoxy resin, and cut into ultrathin sections for electron microscope (H-7100, Hitachi) examination.
Reference:
Wu, S.-X.; Koshimizu, Y.; Feng, Y. P.; Okamoto, K.; Fujiyama, F.; Hioki, H.; Li, Y. Q.; Kaneko, T., and Mizuno, N.: Vesicular glutamate transporter immunoreactivity in the central and peripheral endings of muscle-spindle afferents. Brain Res.,> 1011, 247-251 (2004).
If you decide to try this procedure, we suggest that you complete the Nanogold labeling and silver or gold enhancement, then washing thoroughly before the peroxidase/DAB labeling. This will prevent the reagents used with DAB/peroxidase staining from nucleating silver or gold deposition during enhancement and generating non-specific signal.
However, Gutekunst and co-workers have described a pre-embedding colloidal gold / enzymatic double labeling procedure to label HAP1 and LR11 for electron microscopy in rat and mouse brain tissue in which silver enhancement was performed after DAB development, indicating that DAB does not deposit silver, and widening the options for users of this method. Sections were rinsed in PBS, blocked and incubated in a mixture of primary antibodies HAP1B-C (1:1000) and mLR11 (1:100) overnight, then rinsed in PBS and incubated in a combination of biotinylated donkey anti-rabbit and ultrasmall colloidal gold-conjugated secondary antibody overnight. Sections were rinsed, incubated in avidinbiotin complex, and developed with DAB; then, after postfixation with 2.5% glutaraldehyde, gold particles in sections were intensified using silver enhancement (R-gent SE-EM kit, Aurion). Sections were then further fixed with 0.5% osmium tetroxide in 0.1 M PB for 15 min and processed for electron microscopy.
Reference:
Gutekunst, C. A.; Torre, E. R.; Sheng, Z.; Yi, H.; Coleman, S. H.; Riedel, I. B., and Bujo, H.: Stigmoid Bodies Contain Type I Receptor Proteins SorLA/LR11 and Sortilin. New perspectives on their function. J. Histochem. Cytochem., 51, 841-852 (2003).
More information:
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
HQ Silver has unique advantages for many immunogold-silver experiments, including near-neutral pH and low ionic strength for optimum ultrastructural preservation, and a thickening agent to moderate development and ensure uniform particle size. Giulianini and co-workers provide another demonstration that it may be used with colloidal gold probes from other suppliers: they used HQ Silver-enhanced 5nm colloidal gold to study the immunoreactivity of an antibody to recombinant crustacean hyperglycemic hormone of Nephrops norvegicus, showing that it cross-reacts with neuroendocrine organs of several taxa of malacostracan Crustacea.
Reference:
Giulianini, P. G.; Pandolfelli, N.; Lorenzon, S.; Ferrero, E. A., and Edomi, P.: An antibody to recombinant crustacean hyperglycaemic hormone of Nephrops norvegicus cross-reacts with neuroendocrine organs of several taxa of malacostracan Crustacea. Cell Tissue Res., 307, 243-254 (2002).
Further information on the nature of the interaction of DNA with gold surfaces and nanoparticles is provided by Ganguli and group, who used the atomic force microscope (AFM) to study the interaction of 30nm gold nanoparticles, stabilized with lysine to confer a cationic charge, with linearized plasmid DNA. The authors inferred that the morphology of the complexes is dictated by the relative concentration of the nanoparticles and DNA. A higher concentration of nanoparticles leads to the formation of a network of nanoparticles assembled on DNA, significantly different from the manner in which cationically modified gold nanoparticles of smaller size (<5 nm) arrange linearly on DNA; these constructs are potentially useful models for in vitro studies on the mechanism of DNA condensation, and for developing methods for nanoparticle self-assembly on the DNA template.
Reference:
Ganguli, M.; Babu, J. V., and Maiti, S.: Complex formation between cationically modified gold nanoparticles and DNA: An Atomic Force Microscopic Study. Langmuir, 20, 5165-5170 (2004).
Pivovarova and colleagues use pre-embedding Nanogold® labeling with HQ Silver enhancement highly effectively to localize pre-apoptotic cytochrome c in cultured hippocampal neurons, in a study to characterize the linkage between mitochondrial calcium load and cell vulnerability, and to test the hypothesis that only a subpopulation of mitochondria damaged by N-methyl-D-aspartate (NMDA)-stimulated calcium overload releases apoptogens. By measuring the concentrations of total calcium (free plus bound) in individual mitochondria with a fluorescent indicator, and monitoring parallel structural changes and the subcellular localization of cytochrome c, they found that NMDA stimulation induced dramatic, but mainly reversible, changes in mitochondria, including strong calcium elevation, membrane potential depolarization, and swelling. The discovery that the approximately one-third of mitochondria that were strongly swollen contained elevated matrix calcium, while swelling was absent when Ca2+ entry was abolished, indicate an essential role for Ca overload, and provides support for a support a mechanism in which delayed excitotoxic death involves apoptogen release from a subpopulation of calcium-overloaded mitochondria while other, undamaged mitochondria maintain normal function.
Reference:
Pivovarova N. B.; Nguyen H. V.; Winters C. A.; Brantner C. A.; Smith C. L., and Andrews S. B.: Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J. Neurosci., 24, 5611-5622 (2004).
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
|