|
You might have guessed that you probably need some tweaks in your labeling and microscopy procedure to get the best results from a unique probe like FluoroNanogold™, and here’s where we tell you what to do, and why, and explain how to avoid or fix common problems.
Generally…
If you are using FluoroNanogold™, remember that fluorescent probes and gold probes, on their own, are often used in quite different concentrations. Immunofluorescent conjugates are usually sold in concentrations of 1 mg or so per mL, while gold probes can be 10 to 20 times more dilute. FluoroNanogold™ is packaged and sold at the same concentration as our Nanogold® immunogold conjugates: 80 µg/mL (0.08 mg/mL). Often, immunogold conjugates are used at lower concentrations than immunofluorescent ones: when you are using FluoroNanogold™, the best results are likely to come at a concentration that is somewhere between the two. This is the reason for the two most common issues: low fluorescent signal from the lower fluorophore concentrations, and greater tendency for background, particularly at the electron microscope level. Here’s how to fix these:
Boosting fluorescent signal
Besides the lower concentration, FluoroNanogold™ conjugates also carry fewer fluorophores because they are actually Fab’ fragments, which are only about one-third the size of IgG molecules. In addition, the gold covers up some of the surface space, and there’s probably a little energy transfer from the fluorophores to the gold that cuts into the fluorescence intensity a little. However, these are minor effects and can usually be fixed readily:
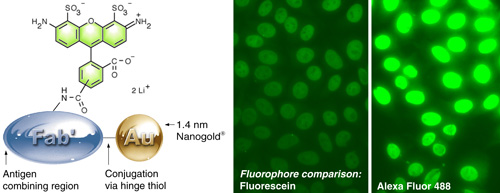
(Left) Alexa Fluor 488 – Fab’ FluoroNanogold.
(Right) Comparison of fluorescein with Alexa Fluor 488 FluoroNanogold™. The same camera exposure was used, to show the difference. Specimen is a positive pattern control slide from a commercial test kit for quantitating human anti-nuclear antibodies (NOVA LiteTM ANA HEp-2) in human serum, stained using the positive control primary antibody, mouse anti-human nuclear antibody secondary, and FluoroNanogold™ - Fab' tertiary probe; both specimens were washed with PBS (30 minutes) between each step. 7 % nonfat dried milk was added to the tetiary antibody solution (original magnification 400 X). |
- Use Alexa Fluor 488 FluoroNanogold™ rather than fluorescein FluoroNanogold™. The Alexa Fluor 488 probe produces dramatically increased fluorescence, as shown above. It is also brighter, more photostable and consistent across a wider pH range, and is imaged with the same filter set.
- Check your camera exposure settings. Manual, rather than automatic, exposure control can let you compensate better for lower fluorescence. Many commercially available immunofluorescent IgG conjugates are larger and more highly labeled, and hence give brighter fluorescence. Automatic exposure adjustment with FluoroNanogold™-stained specimens results in greater exposure, and this can produce higher apparent backgrounds and make it more difficult to separate signal from noise. Setting the camera exposure manually and using a shorter exposure time may allow you to compensate better by adjusting the image properties.
- Balance lower concentrations with longer incubation times: longer times allow greater equilibration of the system, so binding has a chance to maximize.
- Making sure background is as low as possible will also make it easier to compensate by adjusting the image properties or detector sensitivity, or increasing the exposure time.
Background: the usual suspect
This brings us to that old enemy of immunolabeling, background. It is important to recognize that even though some chemical technologies such as hydrophilic, solubilizing coatings are built into Nanogold® to reduce this, FluoroNanogold™ contains not one but two labels, and each brings its own background issues. Fluorophores - even the newer ones like the Alexa Fluor 488, 546 and 594 labels used in FluoroNanogold™ - have some hydrophobic character.
Best Practices
We have investigated background and ways to control it extensively, and developed some best practices for use with FluoroNanogold™. Here is what works best for us:
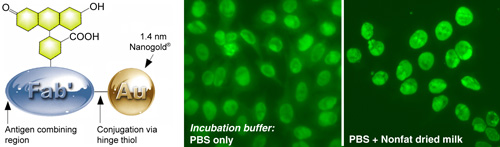
(Left) Fluorescein FluoroNanogold – Fab’.
(Right) Comparison of FluoroNanogold™ incubation buffers.
NOVA LiteTM ANA HEp-2 test kit,1 stained using the positive control primary antibody, mouse anti-human nuclear antibody secondary, and Fluorescein FluoroNanogold™ - Fab' tertiary probe; both specimens were washed with PBS (30 minutes) between each step, then tested with and without 7% nonfat dried milk added to the tetiary antibody solution (original magnification 400 X; same camera exposure time). |
- The most effective blocking agent we have tested is 5% nonfat dried milk. This was found to be particularly effective when mixed with and added to the specimen together with the FluoroNanogold™ conjugate. Cold-water fish gelatin has also been found to be helpful for gold probes in general.
- For reducing the background in electron microscopy, sodium citrate buffer was found to be more effective than other buffers when used as a wash before silver enhancement. 0.02 M sodium citrate at pH 7.0 works well with HQ Silver, while pH 3.5 works best with the Danscher silver formulation.2
- Gold enhancement has been shown to provide very low backgrounds in in situ hybridization and blotting. Therefore, if background is a problem with silver enhancement, we recommend gold enhancement as an alternative.
Troubleshooting
Still seeing it? Check to see if your system has a particular property that predisposes it to background. These include a tendency to hydrophobic interactions, which can occur with membranes; high concentrations of thiols which coordinate strongly to gold (cysteine-rich specimens), or high local ionic charge that can interact with proteins. If so, you can try these fixes:
Hydrophobic interactions: Both the gold and fluorescent labels have some residual hydrophobicity. Adding reagents that reduce hydrophobic interactions to the wash buffer may help remove non-specific binding. Consider using the following:
- 0.6 M triethylammonium bicarbonate buffer (prepared by bubbling carbon dioxide into an aqueous suspension of triethylamine with stirring.3
- 0.1 % to 1 % detergent, such as Tween-20, or Triton X-100. 0.1% saponin may also be useful since its effects are reversible, so ultrastructural preservation may be improved if it is removed in later steps. If you are concerned about membrane integrity, try one of the specialized sugar-based detergents developed for membrane protein crystallization, such as the non-ionic detergents available from Affymetrix (Anatrace).
- 0.1 % to 0.5 % of an amphiphile, such as benzamidine or 1,2,3-trihydroxyheptane.
Thiol-gold interactions: Thiols such as cysteines coordinate strongly to gold, and can displace the surface coating of Nanogold®. If you know you have an unusually high concentration of thiols, try washing with a solution of 50 mM N-ethylmaleimide (NEM) in 20% dimethylsulfoxide – water. NEM reacts with thiols to prevent them from coordinating to gold.
Charge-based interactions: immunoprobes are built on proteins –antibody IgG molecules or Fab’ fragments – and some specimens can have just the right local pH or charge to bind to proteins. Here are a few ways to cut down on charge-charge interactions:
- Increase the ionic strength of your incubation mixture or wash: try adding 0.15 M sodium chloride, or increasing to 0.3 M if you are already working in PBS.
- Adjust the pH – binding interactions may be reduced if you go up or down one or two pH units.
- Try a modified blocking agent, such as an engineered bovine serum albumin.
Need some more help? Please don't hesitate to contact us. We're a working research laboratory, and you'll talk to one of our scientists, who are well-versed in all our products under many applications.
- Tan, E.: Autoantibodies to Nuclear Antigens (ANA): Their Immunobiology and Medicine. Advances in Immunology, 33, 167-240 (1982).
- Powell, R. D.; Halsey, C. M. R.; Spector, D. L.; Kaurin, S. L.; McCann, J., and Hainfeld, J. F. A covalent fluorescent-gold immunoprobe: "simultaneous" detection of a pre-mRNA splicing factor by light and electron microscopy. J. Histochem. Cytochem., 45, 947-956 (1997).
- Safer, D.; Bolinger, L., and Leigh, J. S.: Undecagold clusters for site-specific labeling of biological macromolecules: simplified preparation and model applications. J. Inorg. Biochem., 26, 77-91 (1986).
|
Also in this issue:
|