If you want to really understand a biological system, you need to know how it works at the cellular level, and at the molecular level.
Both require microscopy: light or fluorescence microscopy for cellular features, and – if you really want to see small – electron microscopy for ultrastructural visualization.
However, if you want to label a target and see it both ways, you run into a problem: a lack of labels that show up well in both microscopes.
Until recently, that meant you had to do two separate immunolabeling experiments, and this can create monumental problems in tracing the labeling – or correlating– between the two sets of images.
The two probes might work differently, or intervening processing might distort the specimen so the two images cannot be aligned.
Fortunately Nanoprobes provides a unique solution, with our FluoroNanogold™ combined fluorescent and gold probes.
These immunoprobes include both a fluorescent label and our exclusive 1.4 nm Nanogold® gold nanoparticle label, both linked to a Fab' fragment to give some of the smallest immunoprobes available today. |
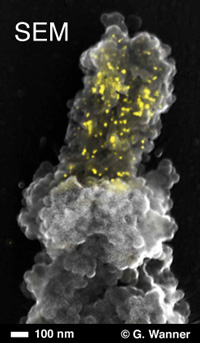
Scanning electron micrograph of peg-like terminal constriction of Oziroë biflora (plant, Hyacinthaceae) chromosome, showing both chromosome topography (secondary electron signal) and hybridized enhanced gold signals (superimposed back-scattered electron signals, yellow) labeling of 45S rDNA in the nucleolus organizing region with Alexa Fluor 488 FluoroNanogold™ -Streptavidin (courtesy of Elizabeth Schroeder-Reiter and Gerhard Wanner).
|
Using FluoroNanogold™ probes, you can label for both fluorescence and electron microscopic observation with a single immunostaining procedure.
As demonstrated by John Robinson and Toshi Takizawa, the fluorescence and gold particle labels show one-to-one correlation. [1] FluoroNanogold™ is a unique probe that lets you correlate optical fluorescence with transmission electron microscopy, [1, 2] scanning electron microscopy, [3] or even X-ray fluorescence [4] to give you several correlative microscopy possibilities.
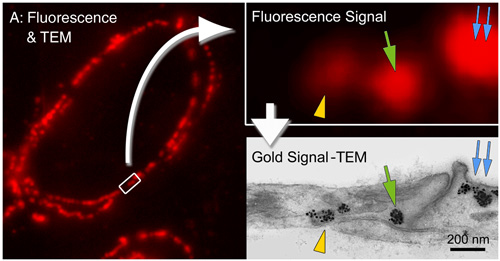
Localization of caveolin-1α in ultrathin cryosection of human placenta using Alexa Fluor 594 FluoroNanogold™-Streptavidin, showing one-to-one correspondence between fluorescent spots and caveola labeled with gold particles. Ultrathin cryosections on formvar film-coated nickel EM grids, incubated with chicken anti-human caveolin-1α IgY for 30 minutes at 37°C, then biotinylated goat anti-chicken F(ab’)2 (13 mg/mL) for 30 minutes at 37°C, then stained with ALEXA-594 FluoroNanogold™-Streptavidin (1:50 dilution) for 30 minutes at room temperature. Non-specific blocked with 1% nonfat dried milk - 5% fetal bovine serum-PBS (figure courtesy of T. Takizawa, Ohio State University, Columbus, OH). |
Try FluoroNanogold™ for these applications:
- Correlate fluorescence data with transmission electron microscopy:
see at the cellular and ultrastructural level. [1,2]
- Check your labeling by fluorescence before processing for electron microscopy. [5]
- Correlative fluorescence microscopy with scanning electron microscopy (SEM);
for highest resolution, use gold enhancement with backscatter detection to see smaller gold in the SEM. [3]
- Correlate optical fluorescence with elemental mapping using X-ray fluorescence. [4]
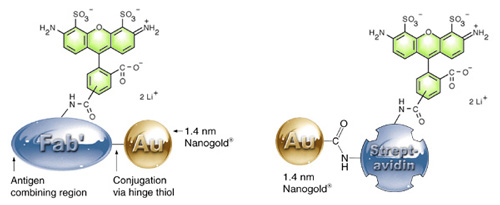
How it works: Alexa Fluor 488 – Fab’ (left) and streptavidin (right) showing the separate, covalent attachment of the 1.4 nm Nanogold® and the Alexa Fluor 488 label to the protein. |
- Takizawa, T.; Suzuki, K., and Robinson, J. M.: Correlative Microscopy Using FluoroNanogold on Ultrathin Cryosections: Proof of Principle; J. Histochem. Cytochem., 46, 1097-1102 (1998).
- Takizawa, T., and Robinson, J. M.: Correlative Microscopy of Ultrathin Cryosections is a Powerful Tool for Placental Research. Placenta, 24, 557-565 (2003).
- Schröder-Reiter, E.; Houben, A.; Grau, J, and Wanner, G.: Characterization of a peg-like terminal NOR structure with light microscopy and high-resolution scanning electron microscopy. Chromosoma, 115, 50-59. (2006).
- McRae, R.; Lai, B.; Vogt, S., and Fahrni, C. J.: Correlative microXRF and optical immunofluorescence microscopy of adherent cells labeled with ultrasmall gold particles. J. Struct. Biol., 155, 22-29 (2006).
- Iliev, A. I.; Ganesan, S.; Bunt, G., and Wouters, F. S.: Removal of pattern-breaking sequences in microtubule binding repeats produces instantaneous tau aggregation and toxicity. J. Biol. Chem., 281, 37195-37204 (2006).
- Ghosh, K.; Nieves, E.; Keeling, P.; Pombert, J. F.; Henrich, P. P.; Cali, A., and Weiss, L. M.: Branching network of proteinaceous filaments within the parasitophorous vacuole of Encephalitozoon cuniculi and Encephalitozoon hellem. Infect Immun., 79, 1374-1385 (2011).
- Abstract from the American society for Microbiology
- Powell, R. D.; Halsey, C. M. R.; Spector, D. L.; Kaurin, S. L.; McCann, J., and Hainfeld, J. F. A covalent fluorescent-gold immunoprobe: "simultaneous" detection of a pre-mRNA splicing factor by light and electron microscopy. J. Histochem. Cytochem., 45, 947-956 (1997).
|
Also in this issue:
|