The Other Electron Tomography:
Nanogold® for EM
We recently discussed how recent advances in cryo-electron tomography methods were creating new opportunities for high-resolution gold labeling in both tissues and isolated protein complexes. Specimen preparation, though, is only half the story: the other half is how you do the EM. A big plus here is the development of electron microscopic tomography methods.[1] These work by collecting data from a specimen that is tilted through a series of angles, then reconstructing the raw data to show the ultrastructure of the specimen, section by section. The advantage is that you can see inside far thicker specimens at high resolution, so you can see both the overall structure of a much larger biological entity, and a labeled target within it at highest resolution.
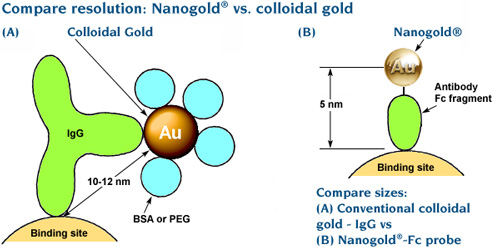
A really small probe, such as Nanogold®, that can penetrate all the way inside a thick specimen, lets you take advantage of these methods, and in this issue we look at some of the results. Theoretical studies indicate that the visibility of small gold labels, such as the 1.4 nm Nanogold® or even Undecagold, is greater in the 3D reconstructions than in the corresponding 2D images.[2] In the real world, this is verified by the use of small Nanogold®-labeled probes that can identify targets with sub-antibody precison,[3-5] and visualized in samples as thick as 250 nm.[4]
Why is Nanogold® ideal for EM tomography?
- Link to almost any probe, not just antibodies or proteins:
- The whole probe is extremely small. Nanogold® is coated with a thin shell of small organic ligands – no extra proteins or polymers needed. The entire label, coating and all, is just 2.5 – 2.7 nm in diameter. This ensures that conjugates have the best chance to penetrate thick sections.
- Those ligands are also tailored for maximum protection and minimum protein affinity – so Nanogold® is highy soluble, won’t stick non-specifically or give background, and is highly biocompatible so it won’t generate an immune response.
- Nanogold® is highly receptive to silver enhancement. Our HQ Silver gives the most uniform, controlled enhancement. Better yet, try gold enhancement with GoldEnhance™ for even greater contrast at smaller particle sizes.
- Chen, X.; Winters, C. A., and Reese, T. S.: Life inside a thin section: tomography. J. Neurosci., 28, 9321-9327 (2008).
- Sousa, A. A.; Aronova, M. A.; Kim, Y. C.; Dorward, L. M.; Zhang, G, and Leapman, R. D.: On the feasibility of visualizing ultrasmall gold labels in biological specimens by STEM tomography. J. Struct. Biol., 159, 507-522 (2007).
- He, W.; Ladinsky, M. S.; Huey-Tubman, K. E.; Jensen, G. J.; McIntosh, J. R., and Björkman P. J.: FcRn-mediated antibody transport across epithelial cells revealed by electron tomography. Nature, 455, 542-546 (2008).
- He, W.; Jensen, G. J., and Björkman, P. J.: Nanogold as a Specific Marker for Electron Cryotomography. Microsc. Microanal., 15, 183-188 (2009).
- Abstract from Cambridge University Press
- Morphew, M.; He, W.; Bjorkman, P. J., and McIntosh, J. R.: Silver enhancement of Nanogold particles during freeze substitution for electron microscopy. J. Microsc., 230, 263-267 (2008).
- Amat, F.; Comolli, L. R.; Nomellini, J. F.; Moussavi, F.; Downing, K. H.; Smit, J., and Horowitz, M.: Analysis of the intact surface layer of Caulobacter crescentus by cryo-electron tomography. J. Bacteriol., 192, 5855-5865 (2010).
|
Also in this issue: |