N A N O P R O B E S E - N E W S
Vol. 11, No. 1 January 31, 2011
Welcome back to our e-Newsletter!
We've got some fresh technical tips, new applications, insider product information and news. We aim to make this a monthly happening and include as much of what you want as possible, so let us know what you'd like to see here.
Thanks!
|
In this issue:
|
Cryomethods benefit from advances in gold labeling as well. With the right gold label, it is possible to pinpoint the localization of targets relative to these new ultrastructures. Small probes, and high-resolution, site-specific labeling are desirable, and Nanoprobes provides a number of reagents and probes to meet this need. Our Nanogold®-Fab’ conjugates, which are only about one-third the size of whole IgG molecules, are the smallest gold probes commercially available and provide higher resolution than colloidal gold, and because Nanogold® does not require any additional macromolecules such as PEG or BSA for stabilization, easily penetrate even to interior sites and restricted spaces within organelles. Furthermore, Nanogold® is also available in reactive forms for labeling other probes, or even for directly labeling sites within protein complexes or organelles. You can use Monomaleimido Nanogold® to label thiol sites (cysteines) or Mono-Sulfo-NHS-Nanogold® to label lysine amines (lysine residues or N-terminal amines).
The application of Nanogold® labeling to cryoelectron microscopy is reviewed in our recent paper in Methods in Enzymology, which contains detailed protocols, tips and tricks both for using our Nanogold® reagents, and their application to cryoelectron microscopy:
Ackerson, C. J.; Powell, R. D., and Hainfeld, J. F.: Site-specific biomolecule labeling with gold clusters. Methods Enzymol., 481, 195-230 (2010).
Abstract (courtesy of Science Direct).
|
|
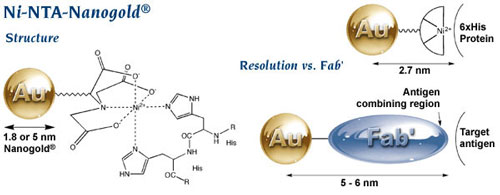
If you are looking at protein complexes and want to label a specific subunit, then even Fab’ can be too big and too disruptive. In that case, how about a gold label which binds to a His tag through a tiny nickel (II) chelate complex? That would be Ni-NTA-Nanogold®, our probe for His-tagged proteins that is now available in 1.8 and 5 nm sizes that are perfect for EM visualization. With its gold-to-target separation of only 1.5 nm or so, you cannot get better resolution – and the binding strength is comparable to antibodies.
|
Ni-NTA-Nanogold® structure and comparison with Fab’;
note significantly higher resolution.
|
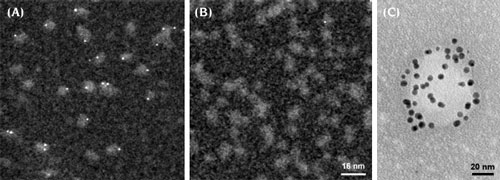 |
Ni-NTA-Nanogold® labeling:
(A) STEM micrograph showing 1.8 nm Ni-NTA-Nanogold® labeling of His-tagged adenovirus A12 knob protein; (B) control without His tags showing no binding; (C) TEM image of T7 virus particle expressing His-tagged capsid protein, decorated with 5nm Ni-NTA-Nanogold®, negatively stained using NanoVan negative stain from Nanoprobes. |
Schraidt and group recently showed how this reagent may be used for high-resolution cryoelectron microscopy in their recent paper in PLoS Pathogens. Many Gram negative pathogens such as Salmonella, Yersinia, or Shigella use the type III secretion system (T3SS) to transfer bacterial toxins to host cells, initiating infections with symptons ranging from mild headaches and diarrhea to life-threatening diseases such as typhoid fever or bubonic plague. A central part of the T3SS is the needle complex, a mega-dalton sized membrane associated complex of several soluble and membrane proteins whose specific roles and organization have hitherto been unclear. The authors used 1.8 nm Ni-NTA-Nanogold® to label the PrgH and PrgK subunits of the needle complex at their N- and C- termini in turn, using subunit proteins with His tags engineered at the terminus under study. They then used cryoelectron microscopy with single particle analysis to average the positions of the gold labels. Careful analysis indicated that the amino terminus of
PrgH and the carboxy terminal domain of PrgK both face the cytoplasm, while the PrgH carboxy terminus is located within the periplasm of the bacterial envelope; the more distinct electron density maximum with C-terminus labeled PrgK indicated that its C-terminal attachment site is more rigid and located closer to the cup of the needle complex. When combined with bacterial genetics, mutational analysis, chemical derivatization and high-resolution mass spectrometry, these results were used to generate a topographic map of a Salmonella typhimurium T3SS needle complex.
Schraidt, O.; Lefebre, M. D.; Brunner, M. J.; Schmied, W. H.; Schmidt, A.; Radics, J.; Mechtler, K.; Gala, J. E.; Marlovits, T. C. and Schmidt, A.: Topology and Organization of the Salmonella typhimurium Type III Secretion Needle Complex Components. PLoS Pathog., 6, e1000824 (2010).
|
Go to Page 2:
Dealing with Disulfides:
Alternatives to DTT or Mercaptoethylamine Reduction
|