Gold enhancement works like silver enhancement: wash, rinse thoroughly, and apply the freshly mixed reagent.
We recommend a few changes from the silver enhancement procedure for best results: the following steps provide the highest sensitivity with the lowest background:
- Incorporate 0.1% Tween-20 (detergent) in the buffers used for blocking, antibody incubation, and washing. This will dramatically reduce background binding. We find that the best washing buffer is TBS-Tween 20: 20 mM Tris pH 7.6, with 150 mM NaCl and 0.1 % Tween-20.
- Include 1% nonfat dried milk (you can use the material sold in supermarkets and food stores) as an additive in the incubation buffer (the buffer in which the Nanogold® is dissolved and applied to the blot) and 5% nonfat dried milk in the blocking buffer used to block specimens before application of antibodies..
Best Results: Beating background
Because gold has a slightly different chemistry to silver, GoldEnhance reagents sometimes show more sensitivity to endogenous reactive sites in biological specimens. This can lead to the development of background: this may be a fine, granular background signal, either general, or localized to specific organelles such as membranes; or it may be uneven or excessively fast development, with the formation of large particles, at target sites or non-specifically.
You can solve these problems by understanding the underlying chemistry and using treatments which neutralize the endogenous reactivity while preserving your specimen. In the sections below, we discuss possible causes and their solutions:
Redox sites within tissue
Like silver enhancement, gold enhancement is a reductive chemical process that is initiated at redox sites, where metal ions can easily be reduced to metal. Endogenous reducing sites that contain thiols (such as cysteine residues) have a high affinity for gold, and thus may bind and reduce gold ions better than they do silver ions. Another problem is transition metal ions which can readily cycle through several different oxidation states: these can promote the deposition of gold from solution and thus the creation of nucleation sites for autometallography.
We suggest that you try these methods to remove reducing sites (use the same procedure for both control and experiment: test in the control first, and if it works in the control, use it for the experiment). Try these suggestions in the order shown, (1) first:
- Wash thoroughly with 0.05 M disodium EDTA (5 minutes), then rinse very thoroughly with water, before applying GoldEnhance™ EM. EDTA is a strong chelating agent that removes metal ions.
- Apply a solution of 50 mM N-ethylmaleimide (NEM) in 20% dimethylsulfoxide – water. NEM reacts with thiols to prevent them from coordinating to gold: this blocks a significant mechanism for binding extraneous gold.
- Wash for 30 seconds with Lugols' iodine (a mixture of iodine and potassium iodide, available from Sigma) either before applying the immunogold probe, or between the immunogold and the GoldEnhance™ EM (rinse very thoroughly with water between this and applying the GoldEnhance™EM). This is an oxidizing agent that has worked well for us in controlling autometallograhic background in in situ hybridization experiments.
- Wash with 1% freshly prepared sodium thiosulfate solution for two minutes before applying the immuogold probe; this is a standard "stop" reaction for silver enhancement and photographic chemistry.
With thick sections or dense materials, the autometallographic reaction may continue in the interior of the specimen even after the reagents have been rinsed off the surface. In this case, use one of the stop reactions given on our technical support page for GoldEnhance.
If you frequently observe background issues, you can tweak the reagent itself. Try these modifications:
- Reduce the development time, to one minute. The specific signal may be sufficiently dense even at the shorter development times, so reducing the time further may remove the background while still giving a strong specific signal.
- When mixing and dispensing the GoldEnhance, use a mixture of 5 parts solution B (activator) to one part solution A (enhancer). Solution B contains a gold stabilizing agent to control the reactivity of gold in solution: by increasing the amount of B, you may inhibit the background while still permitting strong specific deposition.
- Substitute Solution D with 0.05M sodium phosphate with 0.1M sodium chloride, at pH 5.5. This will reduce the pH of the reaction mixture. Generally, autometallographic reactions (silver and gold enhancement) proceed more slowly at lower pH; b reducing the pH, you will slow the gold deposition reaction and gain more control.
Still stuck? Try this fix, developed by Dr. Wanzhong He while he was working in the laboratory of Dr. Pamela J. Bjorkman at the California Institute of Technology, which puts several of these suggestions together. A combination of steps was used to eliminate background:
- A ratio of one part solution A (enhancer) : 5 parts solution B (activator) was used.
- Solution D (buffer) was substituted with 0.05 M sodium phosphate with 0.1 M sodium chloride, pH 5.5.
- Enhancement was performed for 10-15 minutes rather than 3 minutes.
- The reaction was "stopped" using 3% Na2S2O3 for 30 seconds, then washed with 10% acetic acid + 10% glucose for 3 x 5 minutes.
A comparison of results under standard conditions with those using the modified procedure is shown below:
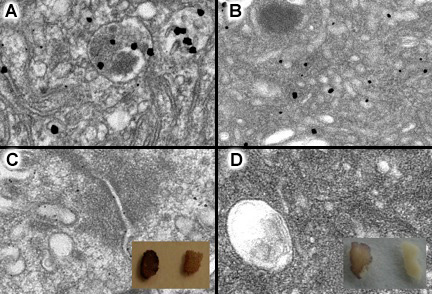
Effect of modified gold enhancement protocol.
Left: Gold enhancement of intestinal tissue with Nanogold® staining. (a) Nanogold® particles, then enhanced with GoldEnhance EM for 3 minutes; (b) control with Nanogold® labeling omitted, showing the granular background. Right: Results using modified procedure described above. (c) Specimen containing Nanogold® particles, enhanced with GoldEnhance EM; (d) control with Nanogold® labeling omitted using identical gold enhancement procedure. Insets show experimental (left) and control (right) tissue (Thanks to Dr. Wanzhong He and Dr. Pamela Bjorkman for the images).