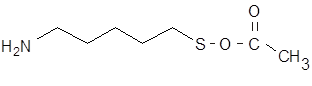
Thioacetylcadaverine In order to determine the location of the gXL (crosslinking) site in fibrinogen and assembled fibrin fibrils, we incorporated an amine donor, thioacetyl cadaverine, into glutamine acceptor sites in fibrinogen in the presence of XIIIa, and then labeled the thiol with undecagold monoaminopropyl maleimide- Au11.
This project had several unique and elegant biochemical and chemical aspects.

3-D atomic model of Au11-thio-cadaverine. It was desired to label the gamma chain crosslinking site in fibrin, which involves residues 398 in the gamma chain of fibrinogen. Plasma transglutaminase (factor XIIIa) (+Ca++) covalently links fibrinogen molecules by formation of e - (g -glutamyl) lysine bonds. The crosslinking site is located in the C-terminal region of the g chain, linking glutamine 398 to lysine 406 on another molecule, thus stabilizing a blood clot.
Since we found that amino gold cluster would not serve as a substrate, we synthesized a 5 carbon chain (cadaverine, 1,5-diaminopentane) with a protected thiol at one end. Cadaverine has been known to serve as a substitute substrate, since it has an amine at the end, mimicking the d-amine of a lysine residue. At the other end was placed a thiol group for linking to gold cluster.
However, free thiol groups frequently cause aggregation, so the thiol group was protected with an acetate group, which was removed just before gold labeling.
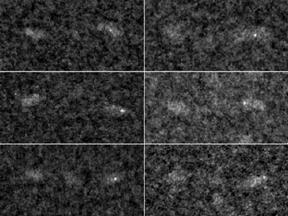
STEM micrograph: Fibrinogen molecules labeled with Au11- cadaverine. Fibrinogen was examined by STEM to locate Au11-cadaverine-labeled gamma 398 D domain sites. These findings show that most C-terminal gamma chains in fibrinogen or fibrin are oriented toward the central domain and indicate that gXL sites in fibrils are situated predominantly between strands, suitably aligned for transverse crosslinking.
|