Other applications
The following paper appeared in Proceedings of the fifty-seventh Annual Meeting, Microscopy Society of America; G. W. Bailey, W. G. Jerome, S. McKernan, J. F. Mansfield, and R. L. Price (Eds.); Springer-Verlag, New York, NY, 1999, pp. 1324-1325. Reproduced by kind permission of Springer-Verlag New York.
A Covalently Linked 10 nm Gold Immunoprobe
Edmund Gutierrez, Richard D. Powell, James F. Hainfeld,* and Peter M. Takvorian**
Nanoprobes, Incorporated, Stony Brook, NY 11790.
* Biology Department, Brookhaven National Laboratory, Upton, NY 11973
** Biological Sciences Department, Rutgers University, Newark, NJ 07102.
Chemically functionalized metal cluster compounds have demonstrated important advantages over colloidal gold as biological microscopy labels. They are covalently cross-linked to the targeting biomolecule, and therefore may be conjugated to a wide range of molecules which cannot be labeled with colloidal gold. The 1.4 nm Nanogold® cluster has been conjugated to peptides,1 lipids2 and oligonucleotides,3 some of which have been proposed as elements of novel molecular wires and novel materials. Dissociation of colloidal gold particles from the conjugate probe, a source of error in quantitative immunogold studies,4 is greatly reduced by covalent cross-linking. Nanogold® is an uncharged molecule, and because its surface is completely coordinated by organic ligands, non-specific binding5 is greatly reduced. Nanogold® conjugates also show greatly enhanced penetration into cells and tissue sections.6 However, gold probes larger than Nanogold® are desirable for improved visualization in specimens with electron-dense regions or staining, or for applications such as double labeling studies with different sized gold labels, or visualizing wider antigen distributions.
We have prepared chemically functionalized, covalently linked 10 nm gold particles, which were conjugated to antibody Fab' fragments to yield highly effective imunogold probes, and may be cross-linked to other probes. 10 nm colloidal gold particles, prepared by the citrate reduction method,8 were stirred with a 10 : 1 mixture of two types of thioalkyl ligands, one functionalized with a solubilizing polyethylene glycol endcap moiety, the other with a protected amino-group. The stabilized nanoparticles were size separated by gel filtration (Superose-6, Pharmacia), deprotected by incubating at 50°C for 1 hour in 0.3N HCl / 20-40% alcohol, and converted to a maleimide derivative with sulfo-succinimidyl 4-[N-maleimido-methyl]cyclohexane-1-carboxylate (Sulfo-SMCC, Pierce). F(ab')2 goat anti-mouse IgG or goat anti-rabbit IgG fragments were reductively cleaved to Fab' fragments using mercaptoethylamine hydrochloride (MEA) which selectively reduces the hinge disulfide bonds to give F(ab')2 fragments, with 5 mm EDTA to prevent reoxidation. Fab' fragments were isolated by gel filtration over a coarse gel (GH25, Amicon) then mixed with the maleimido-gold particles. Conjugates were isolated by gel filtration (Superose-6, Pharmacia).
The Fab' goat anti-rabbit conjugate was used as a secondary probe to label a 43 kDa polar tube protein in spores of the microsporida Glugea americanus, a protozoan parasite which infects the nerves of the American Angler fish, Lophious americanus. The polar tube is a unique apparatus through which infective sporoplasm is transferred to host cells; its identification is diagnostic for microsporida, a class of organisms which are oportunistic in AIDS patients. Fixed G. americanus spores were dehydrated and embedded in LR White resin; thin sections were collected on nickel grids, dried and incubated overnight in a PBS/albumin blocking buffer containing normal goat serum. Grids were incubated for two hours with monoclonal anti-PTP 43 GA antibody, followed by 10 nm gold-Fab' anti-mouse or 12 nm gold-IgG anti-mouse (Jackson) secondary and stained with uranyl acetate. The polar tube was labeled clearly. In immunoblotting experiments against serial dilutions of mouse IgG spotted onto a nitrocellulose membrane, the covalently linked probe showed the same sensitivity as a conventional 10 nm colloidal gold conjugate; however, background staining was significantly lower (Figure 4). Control experiments were conducted in which the conjugation procedure was repeated (a) without converting the gold to maleimide, or (b) without reducing the F(ab')2. In both cases, immunoblot sensitivities were reduced by factors of more than 10, showing that covalent linking is necessary for full probe activity.
- Gregori, L., et al., J. Biol. Chem., 272 (1997) 58.
- Adler-Moore, J., Bone Marrow Transplantation, 14 (1994) S3.
- Alivisatos, A. P., et al., Nature, 382 (1996) 609.
- Behnke, O., et al., Eur. J. Cell Biol., 41 (1986) 326.
- Hainfeld, J. F., Proc. XII Int. Cong. Elec. Microsc. (1990) p. 954-955.
- Takizawa, T., and Robinson, J. O., J. Histochem. Cytochem., 43 (1994) 1615.
- Slot, J. W., and Geuze, H. J., Eur. J. Cell Biol., 38 (1985) 8.
- Ths work was partly supported by SBIR grant number R44 GM50048.
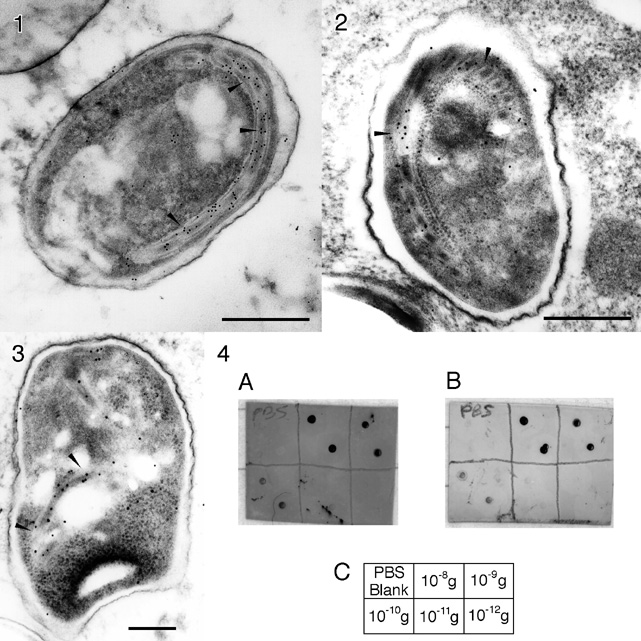
FIGURES 1-3: Electron micrographs of G. americanus spores incubated with monoclonal anti-PTP 43 GA primary antibody with (1) 12 nm colloidal gold anti-mouse secondary (Jackson), and (2) and (3) 10 nm covalent gold-Fab' anti-mouse. Polar tube labeling shown by arrows (bar = 0.5 micrometer).
FIGURE 4: Immunoblot of 10 nm colloidal gold-IgG (A) and 10 nm covalent gold-Fab' (B) anti-mouse conjugate against serial dilutions of mouse IgG spotted onto nitrocellulose membrane; (C) key showing the amounts of mouse IgG in each spot for the corresponding divisions of the blots.
© 1999 Springer-Verlag New York.
Other Applications
|
Product Applications
Research Applications
|
|