Other applications
The following paper appeared in Proceedings of the fifty-seventh Annual Meeting, Microscopy Society of America; G. W. Bailey, W. G. Jerome, S. McKernan, J. F. Mansfield, and R. L. Price (Eds.); Springer-Verlag, New York, NY, 1999, pp. 486-487. Reproduced by kind permission of Springer-Verlag New York.
Gold-Based Autometallography
James F. Hainfeld, Richard D. Powell, Joshua K. Stein, Gerhard W. Hacker,* Cornelia Hauser-Kronberger,** Annie L. M. Cheung,*** and Christian Schöfer$#126;
Nanoprobes, Incorporated, Stony Brook, NY 11790.
* Medical Research Coordination Center, University of Salzburg, Austria.
** Institute of Pathological Anatomy, Salzburg Federal Hospital, Salzburg, Austria.
*** Department of Anatomy, University of Hong Kong, PR China.
~ Institute for Histology and Embryology, University of Vienna, Vienna, Austria.
Gold labels such as Nanogold®1 and colloidal gold2 are enlarged and visualized in the electron microscope or optically by the selective deposition of silver onto their surfaces. This process, known as autometallography (AMG), silver amplification3 or silver enhancement,4 is initiated by exposing the particles to a solution containing silver (I) ions and a reducing agent such as hydroquinone2,3 or n-propyl gallate.4 Particles may be enlarged to between 30 and 100 nm in diameter, giving a distinctive black, punctate staining in the light microscope. Nanogold® labeling with silver amplification is one of the most sensitive methods available for histopathology applications such as in situ hybridization. With Catalyzed Reporter Deposition (CARD; also called Tyramide Signal Amplification, or TSA®; NEN Life Sciences, Boston MA), it has been used to detect as few as 1-2 copies of viral DNA or RNA per cell.5 However, its uses are restricted by reactions of silver (I) with halides and other elements in tissues. Also, after signal development, self-nucleation and non-specific background deposition begin quickly, which can make end-point selection difficult or prevent incorporation into automated procedures.
We have found that gold can also be used as an autometallographic agent. In a suitable chemical environment, it may be selectively deposited onto Nanogold® or colloidal gold to generate a high contrast signal in the electron microscope, and black, punctate staining in the light microscope. It is also effective on blots. We find background staining to be equal to or lower than that found with silver enhancement in several test systems; in addition, although development is complete within 20 minutes, autonucleation is minimal even after two hours exposure to the reagent. This gives the gold autometallographic process a potential advantage for automation. In preliminary electron microscope experiments on the in situ detection of DNA and RNA and the labeling of CD44 in astrocytoma cells,6 particle sizes were found to be more uniform than those found using silver enhancement.
Using gold rather than silver has additional advantages. It can be safely used with osmium tetroxide and uranyl acetate staining, conditions which can etch silver-enhanced gold particles, without the need for protective gold toning. The gold autometallographic reaction is tolerant of a wider range of anions, and may be used in physiological buffers (even with chlorides, which precipitate silver; however, water washes are recommended). In the SEM, gold gives far superior backscatter detection compared with silver. Furthermore, the reaction is less pH sensitive than silver enhancement: the formulation is near neutral, which for some tissues is preferable to the low pH (~3.8) of many silver developers for morphological preservation. In in situ hybridization experiments, such as the detection of HPV-16 viral DNA in SiHa cells using CARD with Nanogold®-streptavidin detection, we find that gold autometallography to be as sensitive as silver amplification, with cleaner backgrounds (Figs. 1-4).
The gold autometallography reagent is prepared from three stable components mixed in equal volume, and applied to specimens for times from five minutes to twenty minutes. A "stop" treatment with 1-2 % sodium thiosulfate for 2 minutes was found to produce cleaner backgrounds in the in situ studies, although a simple water wash to stop development has also been used successfully.
- J. F. Hainfeld and F. R. Furuya, in: "Immunogold-Silver Staining: Principles, Methods and Applications;" M. A. Hayat (Ed.), CRC Press, Boca Raton, FL (1995) 71-96.
- Danscher, G.; Histochemistry 71 (1981) 81.
- Hacker, G. W., et al., J. Histotechnol. 11 (1988) 213.
- Gilerovitch, H. G., et al., J. Histochem. Cytochem. 43 (1995) 337.
- Zehbe, I., et al, Am. J. Pathol., 150 (1997) 1553.
- Ackerley, C. A., unpublished results.
- This work was partly supported by NIH SBIR grant 5R44 GM50048.
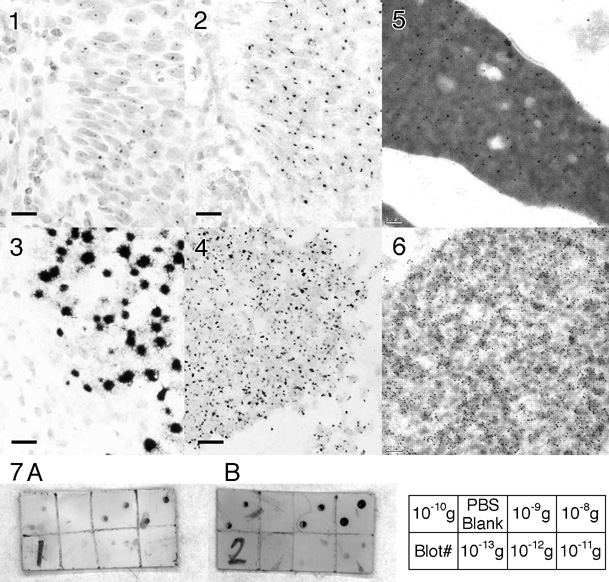
FIGS 1-3: Light micrographs of formalin-fixed serial paraffin sections of cervical carcinoma, in situ hybridized for HPV-16/18 using a biotinylated probe (Pathogene-HPV kit, Enzo) (bar = 10 micrometers). (1) Direct detection using streptavidin-peroxidase; (2) Direct detection using Nanogold®-streptavidin followed by gold autometallography for 18 minutes; (3) Detection using Nanogold®-streptavidin and gold autometallography after CARD amplification.
FIG.4: Formalin-fixed paraffin section from prostate carcinoma biopsy in situ hybridized with a chromosome 12 probe, with CARD-Nanogold®-streptavidin / gold autometallography (bar = 10 micrometers).
FIGS 5-6: Electron micrographs of human testis (bar = 0.1 micrometer). DNA in spermatids was labeled with mouse anti-DNA primary (Roche), then biotinylated anti-mouse antibody (Amersham), followed by (5) Nanogold®-Streptavidin, or (6) goat anti-biotin antibody - 5 nm gold, followed by gold autometallography (8 mins).
FIG. 7: Immunoblot detection of mouse IgG on nitrocellulose using 15 nm gold-goat anti-mouse IgG, enhanced with (A) Silver enhancement (LI Silver, Nanoprobes) and (B) gold autometallography. (C) key showing the amounts of mouse IgG in each spot for the corresponding divisions of the blots.
© 1999 Springer-Verlag New York.
Other Applications
|
Product Applications
Research Applications
|
|