|
The clinical introduction of EnzMet™, which was pending at the time of our recent feature on in situ hybridization, has been approved by the US Food and Drug Administration (FDA).
Ventana Medical Systems, a member of the Roche Group, has announced that the FDA has approved the INFORM HER2 Dual ISH DNA Probe cocktail assay (HER2 Dual ISH) for clinical use in the United States.
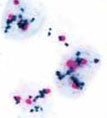 This definitive test for HER2-positive breast cancer clearly identifies patients who can benefit from treatment with the very effective Herceptin (trastuzumab). This definitive test for HER2-positive breast cancer clearly identifies patients who can benefit from treatment with the very effective Herceptin (trastuzumab).
Where previous tests gave a quickly-fading, hard-to-read fluorescent signal (e.g., FISH), Nanoprobes' EnzMet™ technology brings a sharp, permanent black stain that is easily visible with a simple light microscope. The Ventana test adds a contrasting red label for chromosome 17, on which HER2 resides, allowing for a quick control check for extra copies of the HER2 gene --right on the same slide.
This is the first fully automated assay approved by the FDA for determination of HER2 gene status in breast cancer as an aid in the assessment of patients considered for treatment with Herceptin (trastuzumab), a humanized monoclonal antibody that greatly increases the chance of a successful outcome in aggressive breast cancer with an otherwise poor prognosis.
We hope that this is the first of many clinical applications for this versatile and sensitive reagent.
|