Rapid, accurate testing for HER2 breast cancer with EnzMet™
Nanoprobes recently teamed with Ventana Medical Systems to create a definitive HER2 breast cancer test using EnzMet™, applying SISH in automated slide staining for rapid, accurate diagnosis, with significant improvements over FISH and other methods.
In about 30% of all human breast cancers, the HER2 (Human Epidermal Growth Factor Receptor 2) gene is amplified, or duplicated many times; these tumor cells express extra growth factor receptors, stimulating more rapid growth. Luckily, life expectancy with this aggressive, highly malignant type of breast cancer can now be extended with Herceptin, a humanized monoclonal antibody treatment that binds to and blocks this growth factor receptor.
To identify breast cancer patients who would benefit from HER2-targeted Herceptin treatment, an accurate test for HER2 amplification was needed. The Nanoprobes-Ventana HER2 Test successfully provided this vital diagnostic tool.
Using EnzMet™ SISH (Silver In Situ Hybridization), individual HER2 genes are clearly visualized. Amplification is easily observed [1], with a control to distinguish it from duplication of the entire chromosome. What's more, EnzMet™ SISH tests can be archived indefinitely, and require only a standard, bright-field microscope --to clearly pick out a single gene from the other ~25,000 genes in the cell!
With excellent reproducibility shown in two multi-center studies [2,3], Ventana's automated EnzMet™ SISH test is now providing rapid diagnosis, worldwide. Nanoprobes is very proud to be have been a part of this effort.
| EnzMet™ SISH: Silver-Enhanced In Situ Hybridization |
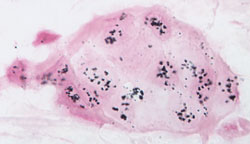
Original magnification x 400; courtesy of Dr. Raymond R. Tubbs, Cleveland Clinic Foundation. |
Human breast cancer biopsy tissue section where single copies (2 per normal cell) of the HER2/neu gene were detected by EnzMet (black spots).
SISH is similar to FISH. However, EnzMet enables bright field detection, a permanent signal, and use of full-strength H&E staining for simultaneous visualization of underlying tissue morphology. |
Learn more about the major advance in cancer diagnosis that EnzMet™ Silver In Situ Hybridization (SISH) has created, in this article from the College of American Pathologists:
"Ventana's HER2 SISH test is fully automated, requiring a six-hour run on its Benchmark series automated stainer, allowing a lab to do an overnight run, for example, and interpret the test the next day. That six-hour turnaround time compares to two to three days to do FISH."
Lusky, Karen. A look at HER2 testing today, HER2 tomorrow. CAP Today, College of American Pathologists, Feb 2008.
Contact Ventana Medical Systems (Roche) about their Silver In Situ Hybridization (SISH) automation system, for rapid testing-- or get manual EnzMet™ SISH for use in your research.
Beyond breast cancer with EnzMet™
Researchers are taking EnzMet™ Silver In Situ Hybridization (SISH) into the fight against other forms of cancer, too.
Two recent studies show that EnzMet™ SISH provides rapid, accurate diagnosis of gastro-esophageal carcinoma [4] and human glioblastoma [5].
Advantages over FISH and other methods:
Both groups report that SISH is faster and easier to use than the conventional fluorescent in situ hybridization procedure (FISH), because SISH development is rapid and can be read using a standard brightfield light microscope. The permanent signal, which does not fade or bleach, was another big advantage.
EnzMet™ SISH can be applied to detect just about any gene or DNA sequence, and therefore could provide superior results in almost any in situ hybridization procedure.
Get EnzMet™ SISH for your own research
The non-automated (manual) version of Silver In Situ Hybridization (SISH) is also available: you can obtain the EnzMet™ HRP Detection Kit for IHC / ISH directly from Nanoprobes.
EnzMet™/SISH can be used to detect nearly any gene or DNA sequence, so it's a great tool for the lab.
References:
- Fritzsche, Florian Rudolf MD; Bode, Peter K. MD; Moch, Holger MD; Kristiansen, Glen MD; Varga, Zsuzsanna MD; Bode, Beata MD. Determination of the Her-2/neu Gene Amplification Status in Cytologic Breast Cancer Specimens Using Automated Silver-enhanced In-situ Hybridization (SISH). American Journal of Surgical Pathology:
August 2010 - Volume 34 - Issue 8 - pp 1180-1185.
- Papouchado BG, Myles J, Lloyd RV, Stoler M, Oliveira AM, Downs-Kelly E, Morey
A, Bilous M, Nagle R, Prescott N, Wang L, Dragovich L, McElhinny A, Garcia CF,
Ranger-Moore J, Free H, Powell W, Loftus M, Pettay J, Gaire F, Roberts C, Dietel
M, Roche P, Grogan T, Tubbs R. Silver in situ hybridization (SISH) for
determination of HER2 gene status in breast carcinoma: comparison with FISH and
assessment of interobserver reproducibility. Am J Surg Pathol. 2010
Jun; 34(6): 767-76.
- Antonino Carbone, Gerardo Botti, Annunziata Gloghini, Gianni Simone, Mauro Truini, Maria Pia Curcio, Patrizia Gasparini, Anita Mangia, Tiziana Perin, Sandra Salvi, Adele Testi, Paolo Verderio. Delineation of HER2 Gene Status in Breast Carcinoma by Silver in Situ Hybridization is Reproducible among Laboratories and Pathologists. The Journal of Molecular Diagnostics, 10 (6) , 527-536, November 2008.
- J. E. Boers, H. Meeuwissen, N. Methorst; Isala Klinieken, Zwolle, Netherlands. HER2 status in 146 gastroesophageal carcinomas assessed by two rabbit monoclonal antibodies (SP3 and 4B5) and two in situ hybridisation methods (FISH and SISH). J Clin Oncol 27, 2009 (suppl; abstr e15555).
- Timo Gaiser, Andreas Waha, Franziska Moessler, Thomas Bruckner, Torsten Pietsch and Andreas von Deimling. Comparison of automated silver enhanced in situhybridization and fluorescence in situ hybridization for evaluation of epidermal growth factor receptor status in human glioblastomas. Modern Pathology (2009) 22, 1263–1271
|
Also in this issue: |