N A N O P R O B E S N E W S
Vol. 11, No. 4 April, 2011
In Situ Hybridization:
The Evolution of Gene Detection,
from Nanogold® to EnzMet™
|
|
Related Nanoprobes products:
|
|
|
This month, having looked at how to attach gold nanoparticles directly to DNA, we take a look at how to detect DNA (and RNA) after it has been put together – by in situ hybridization.
Nanoprobes has been at the forefront of improving in situ hybridization methods with new kits and products, and our article on Nanogold® in situ hybridization remains one of the most visited pages on our website. Recently, we've developed a powerful new technology for in situ hybridization: EnzMet™.
Our in situ hybridization technologies each have their own range of applications; we've gathered here some resources to help you choose the method best suited to your area of research.
Our Human Pathology review paper describes
Nanoprobes in situ hybridization technologies
Human Pathology's feature article discusses the development, features and comparative merits of a number of in situ hybridization detection methods, including:
These in situ hybridization methods are discussed in the context of the development of a chromogenic assay for HER2 gene amplification; this is a significant indicator of malignant tumor behavior in breast cancer, and one of the criteria used to assess patients for humanized monoclonal antibody therapy (Herceptin / Trastuzumab).
| The evolution of metallographic in situ hybridization |
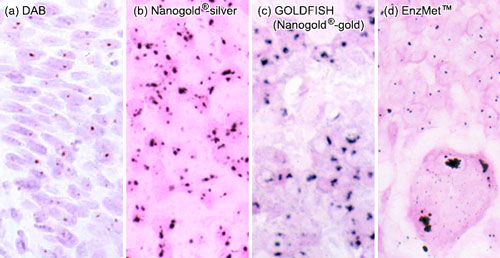 |
(a) and (b): Single copies of HPV-16 in SiHa cells detected by tyramide signal amplification (TSA, also known as CARD, or catalyzed reporter deposition) followed by detection with (a) streptavidin-peroxidase developed with DAB, and (b) streptavidin-Nanogold® with silver acetate autometallography. Copies of HPV-16 appear as single spots. (H & E counterstain. Original magnification X 560).
(c) Nanogold® with gold enhancement (GOLDFISH) procedure in tissue with HER2 gene amplification, showing large, confluent nuclear signals from multiple gene copies in close proximity. Nuclear fast red counterstain (original magnification 400).
(d): EnzMet™ detection of the amplification of individual HER2 gene copies in paraffin-embedded human invasive breast carcinoma biopsy; normal, non-amplified cells contain two copies of the HER2 gene, while the infiltrating HER2-amplified carcinoma cells show multiple copies (original magnification X 400. Image courtesy of Dr. R. R. Tubbs, Cleveland Clinic Foundation). |
- Powell, R. D.; Pettay, J. D.; Powell, W. C.; Roche, P. C.; Grogan, T. M.; Hainfeld, J. F., and Tubbs, R. R.: Metallographic in situ hybridization. Hum. Pathol., 38, 1145-1159 (2007).
- Tubbs, R. R.; Pettay, J.; Skacel, M.; Downs-Kelly, E.; Powell, R. D.; Hicks, D. G., and Hainfeld, J. F.: Gold and Silver-Facilitated Metallographic In Situ Hybridization Procedures for Detection of HER2 Gene Amplification. In: Molecular Morphology in Human Tissues; Hacker G.W., and Tubbs, R. R. (Eds): CRC Press, Boca Raton, FL. Ch. 5, pp. 101-106 (2005).
- Tubbs, R.; Pettay, J.; Hicks, D.; Skacel, M.; Powell, R.; Grogan, T., and Hainfeld, J.: Novel bright field molecular morphology methods for detection of HER2 gene amplification. J. Mol. Histol., 35, 589-594 (2004).
|
Also in this issue: |
|