In our battle against cancer, gold nanoparticles can do more than just show up at the tumor.
The energy that gold absorbs from X-rays to provide such high contrast will also raise the local temperature: this is why implanted wires can cause burns if they are inadvertently X-rayed. But on the micro scale, that heat is useful: it can be used to burn away tumors. High-Z materials absorb X-rays more strongly than tissue, producing highly localized heating which acts to enhance X-ray dose. Because of their high absorption cross-section, gold nanoparticles are good candidates for radiation therapy enhancement. This is one of several therapeutic applications of gold cluster labels and nanoparticles; others include targeted gold nanoparticles used with laser illumination to inactivate cells in a highly selective manner, and radioactive gold conjugates targeted to cancer using antibodies.
At Nanoprobes, we are developing gold-based cancer therapy using the in vivo administration of gold nanoparticles. Vascularization of tumors and increased permeability within tumor vasculature due to angiogenesis provides a natural mechanism for accumulation of gold nanoparticles of specific sizes. At a tumor specifically loaded with gold, the radiation dose would be increased, and radiotherapy would be enhanced.
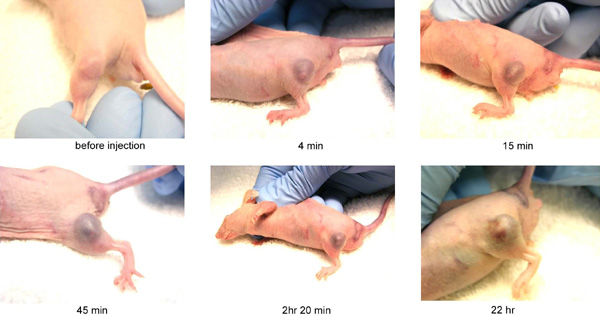
|
| Tumor uptake of gold nanoparticles: accumulation of antibody-targeted 15 nm gold nanoparticles (AuroVist™-15 nm) in implanted mouse tumor. |
Mice bearing EMT-6 mammary carcinomas received a single intravenous injection of 1.9 nm-diameter gold particles (AuroVist™-1.9 nm), up to 2.7 g Au/kg body weight, which elevated concentrations of gold to 7 mg [Au]/g in tumors. Tumor-to-normal-tissue gold concentration ratios remained ~8:1 during several minutes of 250 kVp X-ray therapy. Tumor volume was then monitored over 30 days in four groups of mice: (a) no treatment; (b) gold only (1.35 g Au/kg); (c) irradiation only; and (d) Gold (1.35 g Au/kg) plus irradiation. In the first three groups, tumor volume increased over the course of the experiment, dramatically in the first two groups and more than six-fold in the third; however, in the fourth group, which received both gold and radiation therapy, mean tumor size decreased.
In a separate experiment, long-term (one-year) survival was evaluated for five groups of animals. For those receiving both gold (2.7 g Au/kg) and radiation therapy, the survival rate was 86%, versus only 20% with X-rays alone, and 0% with gold alone:
Long-term cancer survival with NanoGold™/radiation therapy:
| Treatment |
Number of animals |
1-year survival, percent |
| No treatment |
17 |
0 |
| Gold only (1.35 g Au/kg), no irradiation |
4 |
0 |
| Irradiation only (26 Gy, 250 kVp), no gold |
15 |
20% |
| Irradiation after intraveneous injection of 1.35 g Au/kg of gold nanoparticles |
4 |
50% |
| Irradiation after intraveneous injection of 2.7 g Au/kg of gold nanoparticles |
7 |
86% |
The increase in tumors safely ablated was dependent on the amount of gold injected. The gold nanoparticles were apparently non-toxic to mice, and were largely cleared from the body through the kidneys. This novel use of small gold nanoparticles permitted achievement of the high metal content in tumors necessary for significant high-Z radioenhancement.
- Hainfeld, J. F.; Slatkin, D. N., and Smilowitz, H. M.: The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol., 49, N309-N315 (2004).
- Hainfeld, J. F.; Dilmanian, F. A.; Slatkin, D. N., and Smilowitz, H. M.: Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol., 60, 977-985 (2008).
- Hainfeld, J. F.; Dilmanian, F. A.; Zhong, Z.; Slatkin, D. N.; Kalef-Ezra, J. A., and Smilowitz, H. M.: Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Phys. Med. Biol., 55, 3045-3059 (2010).
More information on AuroVist™ and gold nanoparticles in radiotherapy:
|
Also in this issue:
|