![[Nanoprobes Tech Support (14k)]](../Images/logo_tech.gif)
Updated: September 26, 2009
FLUORONANOGOLD* PRODUCT INFORMATION
Fluorescein FluoroNanogoldTM Fab' Conjugates
[Fluorescein FluoroNanogoldTM Fab' Conjugates]

Catalog Numbers &
Product Names::
|
7002 Fluorescein FluoroNanogoldTM-Fab' anti-Mouse IgG
7004 Fluorescein FluoroNanogoldTM-Fab' anti-Rabbit IgG
7055 Fluorescein FluoroNanogoldTM-Fab' anti-Guinea Pig IgG
|
|
Appearance::
|
Fluorescent pale greenish-yellow solution
|
|
Storage::
|
Upon receipt store product at 2-8°C. Product is shipped at ambient temperature.
|
|
Revision::
|
1.8 (September 2009)
|
Technical Assistance Online
![[7002-PDF]](../Images/pdf.gif) Instructions (PDF) Instructions (PDF)
![[7002-PDF]](../Images/pdf.gif) Material Safety Data Sheet (PDF) Material Safety Data Sheet (PDF)
Congratulations on your acquisition of a unique dual labeling immunocytochemical and immunohistochemical reagent: FluoroNanogoldTM. This unique Fab fragment probe contains both the 1.4 nm Nanogold® particle and fluorescein fluorophores, enabling both fluorescence and electron microscope observation of the exact same structures in a single labeling procedure. This probe is smaller than a whole IgG molecule, does not aggregate, and fluorescence quenching due to the gold particle is negligible.
* Patented technology.
- Product Information
- General Considerations for Immunostaining with FluoroNanogoldTM Reagents
- Properties
- Using EM Stains with Fluorescein FluoroNanogoldTM
- Thiol Caution
- Temperature Caution
- Methods:
- Fluorescence Microscopy Immunolabeling with FluoroNanogoldTM
- Electron Microscopy Immunolabeling with FluoroNanogoldTM
- Cells in Suspension
- Thin Sections
- Special Considerations for Direct Viewing of FluoroNanogoldTM in the Electron Microscope
- Silver Enhancement of FluoroNanogoldTM for EM
- Gold Enhancement of FFluoroNanogoldTM for EM
- References
Warning: For research use only. Not recommended or intended for diagnosis of disease in humans or animals. Do not use internally or externally in humans or animals. Non radioactive and non carcinogenic.
FluoroNanogoldTM is a is a unique, dual-purpose probe. FluoroNanogoldTM reagents consist of affinity-purified Fab' fragments (from goat anti-mouse IgG, goat anti-rabbit IgG, and other polyclonal IgG antibodies) labeled with both fluorescein and the 1.4 nm Nanogold particle.1 A fluorescein FluoroNanogoldTM Fab conjugate is shown in Figure 1. In the fluorescence microscope, these probes may be used just like conventional fluorescently-labeled antibodies,2 while in the electron microscope they are visualized in exactly the same manner as for Nanogold® reagents.3 The combination of the fluorescent and electron microscopy, or referred as correlative microscopy12, allows imaging of the same exact structures in both microscopes. The covalent label linkage is stable indefinitely, and the attachment at a hinge thiol site ensures maximum preservation of native immunoreactivity. These reagents are supplied at a concentration of 0.08 mg/mL of Fab' dissolved in 20 mM phosphate buffered saline (150 mM NaCl) at pH 7.4, with 0.1% BSA and 0.05% sodium azide as preservatives. The degree of labeling for each conjugate is typically one Nanogold® particle and 2-3 fluorophores per Fab molecule, and the exact degree of labeling is indicated on the product specification sheet. FluoroNanogoldTM conjugates should be stored at 2-8°C. DO NOT FREEZE. PROTECT FROM LIGHT.
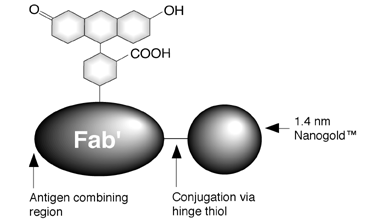
Figure 1: Fab' conjugated to Fluorescein and Nanogold® via a hinge thiol to give FluoroNanogoldTM.
Contents
Basically, normal methodologies for each component of the label may be used successfully with FluoroNanogoldTM labeling agents. Due to some quenching of fluorescence by the gold particle, slightly higher concentrations of antibody are recommended for incubations. A blocking agent of 5% non-fat dried milk has been found to reduce nonspecific background staining in some cases: this should be used before incubation with probe (in standard wash/blocking steps), and additionally, the FluoroNanogoldTM probe can be diluted in a solution also containing 1% non-fat dried milk when it is applied.
- FluoroNanogoldTM contains an extremely uniform 1.4 nm diameter gold particle (±10%).
- The absorption maximum of fluorescein occurs near 494 nm, and the emission maximum is near 518 nm.
- FluoroNanogoldTM-Fab' is smaller than a single whole IgG molecule. It is not significantly larger than Fab'-Nanogold®, the smallest gold immunoprobe commercially available, and will penetrate and reach antigens inaccessible to other gold probes.
- FluoroNanogoldTM-Fab is chromatographically purified through gel filtration columns. There are absolutely no aggregates or other molecular weight impurities. This is in sharp contrast to colloidal gold conjugates which usually are prepared by centrifugation to remove the largest aggregates, and frequently contain smaller aggregates.
- Close to 1 Nanogold® to 1 Fab' make this product distinct from the 0.2 - 10 variable stoichiometry of colloidal gold - antibody preparations.
- FluoroNanogoldTM particles do not have affinity to proteins as do colloidal golds. This reduces background and false labeling.
- FluoroNanogoldTM develops better with silver and gold than do most colloidal golds, giving it higher sensitivity. Both silver and gold enhancement can be used to make the immunolabeling useful for electron microscopy, light microscopy, and immunoblotting with improved results.
Contents
Because the 1.4 nm FluoroNanogoldTM particles are so small, over staining with OsO4, uranyl acetate or lead citrate may tend to obscure direct visualization of individual Nanogold® particles. Four recommendations for improved visibility of FluoroNanogoldTM are:
- Use of reduced amounts or concentrations of usual stains.
- Use of lower atomic number stains such as NanoVanTM, a Vanadium based stain.4
- Enhancement of FluoroNanogoldTM with silver developers, such as LI SilverTM or HQ SilverTM.
- Enhancement of FluoroNanogoldTM with the gold developer, GoldEnhanceTM.
Contents
Nanogold® particles experience loss of gold clusters (Nanogold®) upon exposure to thiols such as beta-mercaptoethanol (BME) or dithiothreitol (DTT). Avoid use of thiol agents. If a reducing environment is needed, reduce the protein, then purify from the thiol agent by column chromatography. Use non-metallic columns, and include 5 mM EDTA with the eluent, since trace metals catalyze thiol oxidation back to disulfides; most thiols do not reoxidize within several hours to several days following this procedure. Then use the FluoroNanogoldTM. If a reducing agent is absolutely required, use a non-thiol agent, such as TCEP (triscarboxyethyl phosphine).
Although Nanogold® is stable under most conditions,5 labeled specimens or conjugates may not be stable above 80°C for long periods. Best results are obtained at room temperature or 4°C. It is best to use silver or gold enhancement before procedures requiring temperatures above 37°C, such as baking, or use low temperature embedding media (e.g., Lowicryl) if labeling before embedding.6
Contents
Several publications describe the successful application of FluoroNanogoldTM for light and electron microscopy. These provide additional protocols, details and applications that may be helpful in obtaining the best results (Refs. 1, 11-15).
If aldehyde-containing reagents have been used for fixation, these should be quenched before labeling. This may be achieved by incubating the specimens for 5 minutes in 50 mM glycine solution in PBS (pH 7.4). Ammonium chloride (50 mM) or sodium borohydride (0.5 - 1 mg/mL) in PBS may be used instead of glycine.
The procedure below2 describes an example of the use of a FluoroNanogoldTM conjugate as a secondary antibody probe. Dilutions of FluoroNanogold will vary with different procedures, but a final concentration of 0.2-10 µg/mL (1 : 8 to 1 : 400 dilution) is advisable as a starting point for most applications; for simultaneous electron microscopy labeling, a compromise between the optimum concentrations for fluorescence and electron microscopy maybe necessary. Other protocols and techniques used with fluorescently labeled antibodies may also be used withFluoroNanogoldTM. It should also be noted that the fluorescence intensity of fluorescein is pH-dependent: it is maximized at pH 9.0 or higher, reducing to approximately 85% at pH 7 and decreasing rapidly at lower pH values. Therefore, we recommend that the buffer used for the final wash should have a pH value of 7.4 or higher.
- Fix cells in freshly-prepared 2 % formaldehyde in PBS for 15 mins at 20°C; alternatively, fix in 100% methanol at -20°C for 3 minutes; if methanol fixation is used, skip to step 4.
- Wash in PBS (3 x 10 mins).
- Permeabilize in 0.2% Triton X-100 plus 1% normal serum (NS) from the host species of theFluoroNanogoldTM-conjugated antibody in PBS at pH 7.3 for 5 minutes on ice.
- Wash in PBS with 1% NS (3 X 10 mins).
- Incubate in the appropriate concentration of primary antibody for 1 hour at room temperature in a humidified chamber. If using 22 mm X 22 mm square cover slips, 30 µL of diluted antibody is placed on the coverslip and the coverslip is inverted onto a glass slide. The slide is then placed in a humidified chamber which is incubated at room temperature.
- Wash in PBS with 1% NS + 5% non-fat dried milk (3 X 10 mins).
- Incubate with FluoroNanogoldTM reagent at a final concentration of 0.2-10 ?g/mL (1 : 8 to 1 : 400 dilution), diluted in buffer containing 1% non-fat dried milk, for 1 hour in a humidified chamber at room temperature.
- Wash in PBS (4 X 10 mins).
- Mount coverslip with a drop of mounting medium. Observe as usual.
PBS Buffer:
- 20 mM phosphate
- 150 mM NaCl
- pH 7.4
Contents
The procedures given in this section are complete immunolabeling procedures, and are also recommended for Nanogold® conjugates. If the specimen has already been labeled and observed by fluorescence microscopy, it requires only mounting, silver or gold enhancement (if necessary) and negative staining according to your usual electron microscopy protocol before observation.
If aldehyde-containing reagents have been used for fixation, these must be quenched before labeling. This may be achieved by incubating the specimens for 5 minutes in 50 mM glycine solution in PBS (pH 7.4). Ammonium chloride (50 mM) or sodium borohydride (0.5 - 1 mg/mL) in PBS may be used instead of glycine.
Cells in Suspension
If the cells are already labeled, mount, stain and observe as usual. If a different specimen is to be used, the procedure below is recommended:
- Optional fixing of cells: e.g., with glutaraldehyde (0.05 - 1% for 15 minutes) in PBS. Do not use Tris buffer since this contains an amine which reacts with glutaraldehyde.
- Centrifuge cells (e.g. 1 mL at 107 cells/ml) at 300 X g, 5 minutes; discard supernatant; resuspend in 1 mL buffer. Repeat this washing (centrifugation and resuspension) 2 times.
- Incubate cells with 0.02 M glycine in PBS (5 mins). Centrifuge, then resuspend cells in PBS-Milk buffer (specified below) or PBS containing 1% BSA for 5 minutes.
- Place 50 - 200 µL of cells into Eppendorf tube and add 5 - 10 µL of primary antibody (or antiserum). Incubate 30 minutes with occasional shaking (do not create bubbles which will denature proteins).
- Wash cells using PBS-Milk as described in step 2 (2 X 5 mins). Resuspend in 1 mL PBS-Milk buffer.
- Dilute FluoroNanogoldTM to a final concentration of 0.2-10 µg/mL (1 : 8 to 1 : 400 dilution) in PBS-1% Milk buffer and add 30 µL to cells; incubate for 30 minutes with occasional shaking.
- Wash cells in PBS buffer as described in step 2 (2 X 5 mins).
- Fix cells and antibodies using a final concentration of 1% glutaraldehyde in PBS for 15 minutes. Then remove fixative by washing with PBS buffer (3 X 5 mins).
|
PBS-Milk Buffer:
|
PBS Buffer:
- 20 mM phosphate
- 150 mM NaCl
- pH 7.40
|
Negative staining
Negative staining may be used for electron microscopy of small structures or single molecules which are not embedded. Negative stain must be applied after the silver enhancement. NanoVanTM negative stain is specially formulated for use with Nanogold® reagents;4 it is based on vanadium, which gives a lighter stain than uranium, lead or tungsten-based negative stains and allows easier visualization of FluoroNanogoldTM particles with little or no silver enhancement.
Thin Sections
Labeling with FluoroNanogoldTM may be performed before (the pre-embedding method)7,8 or after embedding and sectioning (the post-embedding method).7,8 The procedures for both methods are described below.
Thin sections mounted on grids are floated on drops of solutions on parafilm or in well plates. Hydrophobic resins usually require pre-etching.
PROCEDURE FOR PRE-EMBEDDING METHOD:7
If specimen has already been labeled with FluoroNanogoldTM, skip to step 9. If a fresh specimen is required for EM, the following procedure is recommended.
- Float on a drop of water for 5 - 10 minutes.
- Incubate cells with 1% bovine serum albumin in PBS buffer at pH 7.4 for 5 minutes; this blocks non-specific protein binding sites and minimizes non-specific antibody binding.
- Incubate with primary antibody, diluted at usual working concentration in PBS-1% Milk buffer or PBS containing 1% BSA (1 hour or usual time. Buffer formulations are given below).
- Rinse with PBS-Milk (3 X 1 min).
- Incubate with FluoroNanogoldTM reagent diluted to a final concentration of 0.2-10 µg/mL (1 : 8 to 1 : 400 dilution) in PBS - 1% Milk with 1% normal serum from the same species as the FluoroNanogoldTM reagent, for 10 minutes to 1 hour at room temperature.
- Rinse with PBS- Milk (3 X 1 min), then PBS (3 X 1 min).
- Postfix with 1% glutaraldehyde in PBS (10 mins).
- Rinse in deionized water (2 X 5 min).
- Perform silver or gold enhancement (e.g., HQ SilverTM or GoldEnhanceTM), as specified in those product directions.
- Dehydrate and embed according to usual procedure.
- Stain (uranyl acetate, lead citrate or other staining reagent) as usual before examination.
Silver enhancement may be performed before or after embedding (see below); it should be completed before postfixing or staining with osmium tetroxide, uranyl acetate or similar reagents is carried out.
PROCEDURE FOR POST-EMBEDDING METHOD:7
- Prepare sections on plastic or carbon-coated nickel grid. Float on a drop of water for 5 - 10 minutes.
- Incubate with 1% solution of bovine serum albumin in PBS buffer at pH 7.4 for 5 minutes to block non-specific protein binding sites.
- Incubate with primary antibody, diluted at usual working concentration in PBS-1% Milk buffer or PBS containing 1% BSA (1 hour or usual time. Buffer formulations are given below)
- Rinse with PBS-Milk (3 X 1 min).
- Incubate with Fluorescein FluoroNanogoldTM reagent diluted to a final concentration of 0.2-10 µg/mL (1 : 8 to 1 : 400 dilution) in PBS - 1% Milk with 1% normal serum from the same species as the FluoroNanogoldTM reagent, for 10 minutes to 1 hour at room temperature.
- Rinse with PBS (3 X 1 min).
- Postfix with 1% glutaraldehyde in PBS at room temperature (3 mins).
- Rinse in deionized water for (2 X 5 min).
- If desired, contrast sections with uranyl acetate and/or lead citrate before examination.
Silver or gold enhancement may also be used to render theFluoroNanogoldTM particles more easily visible (see below); this is recommended if stains such as uranyl acetate or lead citrate are applied. Silver enhancement should be completed before these stains are applied.
|
PBS-Milk Buffer:
|
PBS Buffer:
- 20 mM phosphate
- 150 mM NaCl
- pH 7.40
|
Contents
For most work, silver or gold enhancement is recommended to give a good signal in the electron microscope (see below). For particular applications, visualization of theFluoroNanogoldTM directly may be desirable. Generally this requires very thin samples and precludes the use of other stains.
FluoroNanogoldTM provides a much improved resolution and smaller probe size over other colloidal gold antibody products. However, because FluoroNanogoldTM is only 1.4 nm in diameter, it will not only be smaller, but will appear less intense than, for example, a 5 nm gold particle. With careful work, however, FluoroNanogoldTM may be seen directly through the binoculars of a standard EM even in 80 nm thin sections. However, achieving the high resolution necessary for this work may require new demands on your equipment and technique. Several suggestions follow:
- Before you start a project with FluoroNanogoldTM it is helpful to see it so you know what to look for. Dilute the FluoroNanogoldTM stock 1:5 and apply 4 µL to a grid for 1 minute. Wick the drop and wash with deionized water 4 times.
- View FluoroNanogoldTM at 100,000 X magnification with 10 X binoculars for a final magnification of 1,000,000 X. Turn the emission up full and adjust the condenser for maximum illumination.
- The alignment of the microscope should be in order to give 0.3 nm resolution. Although the scope should be well aligned, you may be able to skip this step if you do step 4.
- Objective stigmators must be optimally set at 100,000 X. Even if the rest of the microscope optics are not perfectly aligned, adjustment of the objective stigmators may compensate and give the required resolution. You may want to follow your local protocol for this alignment but since it is important, a brief protocol is given here:
- At 100,000 X (1 X 106 with binoculars), over focus, under focus, then set the objective lens to in focus. This is where there is the least amount of detail seen.
- Adjust each objective stigmator to give the least amount of detail in the image.
- Repeat steps a and b until the in focus image contains virtually no contrast, no wormy details, and gives a flat featureless image.
- Now underfocus slightly, move to a fresh area, and you should see small black dots of 1.4 nm size. This is the Nanogold®. For the 1:5 dilution suggested, there should be about 5 to 10 gold spots on the small viewing screen used with the binoculars. Contrast and visibility of the gold clusters is best at 0.2 - 0.5 m defocus, and is much worse at typical defocus values of 1.5 - 2.0 m commonly used for protein molecular imaging.
- In order to operate at high magnification with high beam current, thin carbon film over fenestrated holey film is recommended. Alternatively, thin carbon or 0.2% Formvar over a 1000 mesh grid is acceptable. Many plastic supports are unstable under these conditions of high magnification/high beam current and carbon is therefore preferred. Contrast is best using thinner films and thinner sections.
- Once you have seen Nanogold® you may now be able to reduce the beam current and obtain better images on film. For direct viewing with the binoculars reduction in magnification from 1,000,000 X to 50,000 X makes the Nanogold® much more difficult to observe and not all of the golds are discernable. At 30,000 X (300,000 X with 10 X binoculars) Nanogold® particles are not visible. It is recommended to view at 1,000,000 X, with maximum beam current, align the objective stigmators, and then move to a fresh area, reduce the beam, and record on film.
- If the demands of high resolution are too taxing or your sample has an interfering stain, a very good result may be obtained using silver enhancement to give particles easily seen at lower magnification.
Contents
FluoroNanogoldTM will nucleate silver deposition resulting in a dense particle 2-80 nm in size or larger depending on development time. If specimens are to be embedded, silver enhancement is usually performed after embedding, although it may be done first. It must be completed before any staining reagents such as osmium tetroxide, lead citrate or uranyl acetate are applied, since these will nucleate silver deposition in the same manner as gold and produce non-specific staining. With FluoroNanogoldTM reagents, low-temperature resins (e.g. Lowicryl) should be used and the specimens kept at or below room temperature until after silver development has been completed. Silver development is recommended for applications of FluoroNanogoldTM in which these stains are to be used, otherwise the FluoroNanogoldTM particles may be difficult to visualize against the stain.
Our LI SilverTM silver enhancement system is convenient and not light sensitive, and suitable for all applications. Improved results in the EM may be obtained using HQ SilverTM, which is formulated to give slower, more controllable particle growth and more uniform particle size distribution.9
Specimens must be thoroughly rinsed with deionized water before silver enhancement reagents are applied. This is because the buffers used for antibody incubations and washes contain chloride ions and other anions which form insoluble precipitates with silver. These are often light-sensitive and will give non-specific staining. To prepare the developer, mix equal amounts of the enhancement components immediately before use. FluoroNanogoldTM will nucleate silver deposition resulting in a dense particle 2-20 nm in size or larger depending on development time. Use nickel grids (not copper).
Fluorescence microscopy should be performed BEFORE silver enhancement. This is because the fate of the fluorophores during silver enhancement has not been determined; deposition of silver may obscure the fluorescence.
The relevant procedure for immunolabeling should be followed up to step 7 as described above. Silver enhancement is then performed as follows:
- Rinse with deionized water (2 X 5 mins).
- OPTIONAL (may reduce background)1: Wash several times with 0.02 M sodium citrate buffer, pH 7.0.
- Float grid with specimen on freshly mixed developer for 1-12 minutes, or as directed in the instructions for the silver reagent. More or less time can be used to control particle size. A series of different development times should be tried, to find the optimum time for your experiment.
- Rinse with deionized water (3 X 1 min).
- Mount and stain as usual.
Fixing with osmium tetroxide may cause some loss of silver; if this is found to be a problem, slightly longer development times may be appropriate. Alternatively, use of 0.1% osmium tetroxide instead of 1% has been found to give similar levels of staining while greatly reducing etching of the silver particles.
NOTE: Treatment with osmium tetroxide followed by uranyl acetate staining can lead to much more drastic loss of the silver enhanced Nanogold® particles. This may be prevented by gold toning:10
- After silver enhancement, wash thoroughly with deionized water.
- 0.05% gold chloride: 10 minutes at 4°C.
- Wash with deionized water.
- 0.5% oxalic acid: 2 mins at room temperature.
- 1% sodium thiosulfate (freshly made) for 1 hour.
- Wash thoroughly with deionized water and embed according to usual procedure.
Contents
The small 1.4 nm Nanogold® particles may alternatively be enhanced (grown to a larger size) for better visibility using GoldEnhanceTM EM. Gold enhancement may be preferable to silver enhancement in some cases, due to the different properties of GoldEnhanceTM EM which provide advantages over silver enhancement:
- Gold is chemically more stable and is not depleted by osmium or uranyl stains.
- Gold has higher backscattering and is useful for SEM.
- GoldEnhanceTM EM is not light insensitive it can be used in normal room lighting, and development followed in the light microscope.
- GoldEnhanceTM EM may be used with physiological buffers, such as ones containing chloride, which precipitates silver enhancers.
Gold Enhancement follows a similar procedure to silver enhancement, but for specific directions, see the instructions provided with GoldEnhanceTM EM.
Contents
- Powell, R. D.; Halsey, C. M. R., and Hainfeld, J. F.: Combined fluorescent and gold immunoprobes: Reagents and methods for correlative light and electron microscopy. Micros. Res. Technique, 42, 2-12 (1998); Powell, R. D.; Halsey, C. M. R.; Spector, D. L.; Kaurin, S. L.; McCann, J., and Hainfeld, J. F.: A covalent fluorescent-gold immunoprobe: "simultaneous" detection of a pre-mRNA splicing factor by light and electron microscopy. J. Histochem. Cytochem., 45, 947-956 (1997); Robinson, J. M., and Vandré, D. D.: Efficient immunocytochemical labeling of leukocyte microtubules with FluoroNanogold: An important tool for correlative microscopy. J. Histochem. Cytochem., 45, 631-642 (1997).
- Spector, D. L., and Smith, H. C.: Redistribution of U-snRNPs during mitosis. Exp. Cell Res., 163, 87-94 (1986).
- Hainfeld, J. F., and Powell R. D.: Nanogold Technology: New Frontiers in Gold Labeling. Cell Vision, 4, 308-324 (1997); Furuya, F. R., and Hainfeld, J. F.: A 1.4nm Gold cluster covalently attached to antibodies improves immunolabeling. J. Histochem. Cytochem., 40, 177-184 (1992); Furuya, F. R.; Hainfeld, J. F., and Powell, R. D.: A new 1.4 nm Gold-Fab Probe. Proc. 49th Ann. Mtg., Electron. Micros. Soc. Amer.; Bailey, G. W. (Ed.), San Francisco Press, San Francisco, CA, p. 284 (1991).
- Tracz, E.; Dickson, D. W.; Hainfeld, J. F., and Ksiezak-Reding, H. Brain Res., 773, 33-44 (1997); Gregori, L.; Hainfeld, J. F.; Simon, M. N., and Goldgaber, D.: Binding of amyloid beta protein to the 20S proteasome. J. Biol. Chem., 272, 58-62 (1997); Hainfeld, J. F.; Safer, D.; Wall, J. S.; Simon, M. N.; Lin, B. J., and Powell, R. D.: Methylamine Vanadate (NanoVan) Negative Stain. Proc. 52nd Ann. Mtg., Micros. Soc. Amer.; G. W. Bailey and Garratt-Reed, A. J., (Eds.); San Francisco Press, San Francisco, CA, p. 132 (1994).
- Hainfeld, J. F., and Furuya, F. R.: Silver-enhancement of Nanogold and undecagold. in Immunogold-Silver Staining: Principles, Methods and Applications (M. A. Hayat, Ed.), CRC Press, Boca raton, FL., 1995: pp. 71-96.
- Krenács, T., and Krenács, L.: Comparison of embedding media for immunogold-silver staining. in Immunogold-Silver Staining: Principles, Methods and Applications (M. A. Hayat, Ed.), CRC Press, Boca raton, FL., 1995: pp. 57-69.
- J. E. Beesley, in Colloidal Gold: Principles, Methods and Applications (M. A. Hayat, Ed.), Academic Press, New York, 1989; Vol. 1, pp. 421-425.
- Lujan, R.; Nusser, Z.; Roberts, J. D. B.; Shigemoto R.; Ohishi, H., and Somogyi, P.: Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J. Chem. Neuroanat., 13, 219-241 (1997).
- Humbel, B. M.; Sibon, O. C. M.; Stierhof, Y.-D., and Schwarz, H.: Ultra-small gold particles and silver enhancement as a detection system in immunolabeling and In Situ hybridization experiments. J. Histochem. Cytochem., 43, 735-737 (1995).
- Arai, R.; Geffard, M., and Calas, A.: Intensification of labelings of the immunogold silver staining method by gold toning. Brain Res. Bull., 28, 343-345 (1992).
- Robinson, J. M.; Takizawa, T.; Pombo, A., and Cook, P. R.: Correlative fluorescence and electron microscopy on ultrathin cryosections: bridging the resolution gap. J. Histochem. Cytochem., 49, 803-808 (2001).
- Robinson, J. M.; Takizawa, T., and Vandré, D. D.: Applications of gold cluster compounds in immunocytochemistry and correlative microscopy: comparison with colloidal gold. J. Microsc., 199, 163-179 (2000).
- Takizawa, T., and Robinson, J. M.: FluoroNanogold is a bifunctional immunoprobe for correlative fluorescence and electron microscopy. J. Histochem. Cytochem., 48, 481-486 (2000).
- Takizawa, T., and Robinson, J. M.: Analysis of antiphotobleaching reagents for use with FluoroNanogold in correlative microscopy. J. Histochem. Cytochem., 48, 433-436 (2000).
- Takizawa, T., Suzuki, K., and Robinson, J. M.: Correlative microscopy using FluoroNanogold on ultrathin cryosections. Proof of principle. J. Histochem. Cytochem., 46, 1097-1102 (1998).
Technical Assistance Available.
For a complete list of references citing this product, please visit our References page.
Contents
|