Abdominal aortic aneurysm (AAA) is a hidden threat, lurking behind an asymptomatic progression. A potential key to predicting the disease was recently investigated, by Dr. Bram Trachet and his team at the bioMMeda research group at Ghent University in Belgium. Using AuroVist™, a non-toxic x-ray contrast agent for micro-CT, they created detailed 3D images of the aorta in a mouse model over time, tracking aneurysms as they formed. Points of blood flow turbulence in the aorta, calculated with the help of ultrasound scans, were found to have qualitative correlation to areas where aortic bulging later occurred. "We cannot exclude that hemodynamics play a role in the initial phases of AAA formation," the group concluded.
3D mapping of aortic changes over time in live animals
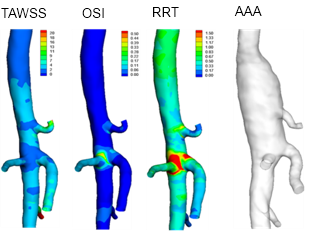
Day 1 baseline calculations of time-averaged wall shear stress (TAWSS), oscillatory shear index (OSI) and relative residence time (RRT) as obtained from computational fluid dynamics, vs. aneurysm development at right (AAA) at day 31. Volumetric renderings from in vivo micro-CT of mouse aorta after IV injection of AuroVist™ 15 nm.
Mouse aortic hemodynamics – the mechanics of flow within blood vessels – were studied using computational fluid dynamics simulations based on in vivo measurements. This is challenging because it requires imaging at high resolution to accurately map both the 3-dimensional geometry of the arteries, and measuring the rates of blood flow at each point.
Previous research was limited by the lack of in vivo imaging methods needed for the arterial measurements in mice. Trachet and group overcame this by using Nanoprobes' AuroVist™ 15 nm, which provided vascular contrast without toxicity --allowing 'in vivo vascular casting', imaging of the vascular system in living, anesthetized mice, with repeated application over the course of the study.
These data were then combined with high-frequency ultrasound measurements to provide a data set for the flow through the abdominal aorta. Using computational fluid dynamics, the group was able to map locations of disturbed blood flow, which might negatively affect the aortic endothelium.
Finding a missing baseline: a mouse model allows study of an 'undetectable' condition
A particular problem with predicting abdominal arterial aneurysm is that the condition is usually asymptomatic until a catastrophic rupture occurs. It is therefore virtually impossible to monitor its genesis and progression in humans because the baseline data are missing. However, an appropriate animal model can give investigators a window into the process.
Trachet and group modeled aneurysm formation using male ApoE -/- mice, implanted with an osmotic pump that was used to deliver Angiotensin II over a 28-day period. Ultrasound and micro-CT data were acquired at baseline (before pump implantation) and again at end-stage (31 days after pump implantation). For the micro-CT observation, the mice were injected intravenously with 100 microliters (20 mg Au) of Aurovist™-15 nm gold nanoparticle contrast agent, then imaged at 70 kVp.
Computational fluid dynamic simulation indicated that a local region of disturbed blood flow coincided qualitatively with the induced aneurysm in three out of four mice, although further detailed analysis did not support the relationship. From these partial indications, however, hemodynamics remain a possible predictor of aneurysm formation, and the relationship warrants further research. Crucially, the group has defined an experimental-computational framework for future study of the disease and its elusive developmental factors in mice, and a better understanding of this disease may someday provide a way to identify individuals most at risk for this life-threatening condition.
Bringing computationally-enhanced visualization to the study of cardiovascular problems
Dr. Trachet and his colleagues (Bols, 2013) have since added another computational tool to the analysis of aortic aneurysm, pioneering a method to compute the initial stress distribution present in a scanned aneurysm, thus accounting for the stresses present in a scanned geometry due to the in vivo blood pressure. They again used intravenous injection of AuroVist™ 15 nm to achieve high-resolution 3D images of AAA in a mouse model, upon which their computational backward displacement method was successfully performed to determine the unloaded geometry, which was then used to recover the scanned geometry, but this time including its initial stress distribution . Their contribution allows for a more correct computational analysis of mouse-specific stresses based on geometries obtained from in vivo scans, and may be an important step toward a more accurate understanding of the pathophysiology of cardiovascular disease.
"This is exciting work," said Dr. James F. Hainfeld, founder of Nanoprobes, the maker of AuroVist™. "They're moving us closer to understanding aneurysm formation; it gives us hope for one day detecting them early."