Areas of increased blood volume and vascular permeability have long been recognized as key indicators of tumor growth, and spotting them is crucial for both cancer diagnosis and treatment planning. New research has found a way to clearly see these two vital signs in one color-coded image, using the unique characteristics of AuroVist™ gold nanoparticles and iodine for contrast, and a specially tuned, dual energy CT scan for each.
Since tumors are growing, they call on the body to create lots of new blood vessels, in and around them. New blood vessels are leaky, so anything in the bloodstream of a particular size will pass through and accumulate in a tumor, but stay out of healthy areas. This Enhanced Permeability and Retention effect (EPR) is just beginning to be used as an important delivery method to tumors, and the degree of leakiness has been found to be a key indicator of tumor growth rate.
At Duke University's Center for In Vivo Microscopy, Dr. Cristian Badea and his colleagues saw EPR as an opportunity to deliver a substance to "light up" on a CT scan, to give a visual indication of the angiogenic leakiness of a tumor and thereby its growth rate. They chose the x-ray contrast agent AuroVist™ from Nanoprobes, which at 15 nm was perfectly sized, and as a gold nanoparticle would show up clearly on a CT scan tuned to the appropriate energy.
"We're excited to see AuroVist being used to visualize tumor growth," said Dr. James F. Hainfeld, founder and chief research scientist at Nanoprobes. "We engineered the particle as part of our cancer research, where we delivered AuroVist to brain tumors via EPR for radiation enhancement, so it's perfect for this purpose."
To complement the gold nanoparticle measurement of EPR, Badea's lab turned to a much larger liposomal iodine, which would instead stay in the bloodstream. A CT scan tuned for iodine will then reveal the blood vessels in the tumor, giving a visual indicator of growth in terms of vascular proliferation.
Just published in Physics in Medicine and Biology, their technique yielded amazingly clear images that give a direct visualization of tumor growth. These results in lab animals are a crucial step toward better cancer diagnosis and treatment in humans.
Under the hood:
Complementary X-ray attenuation profiles
enable the separation of gold from iodine by micro-CT
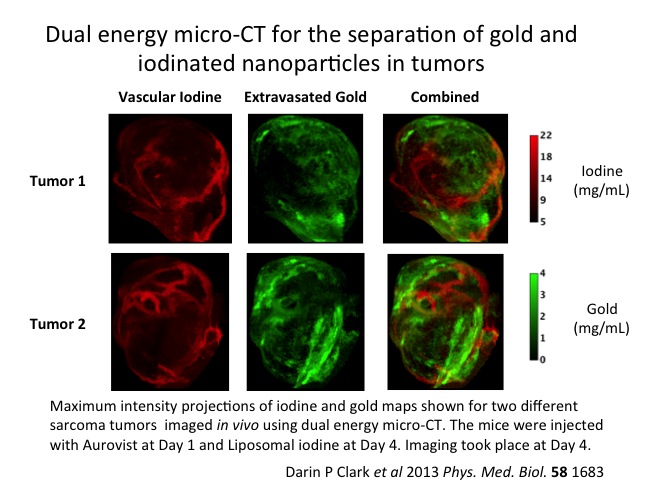
In their study, the tumor was imaged using two polychromatic X-ray sources, and contrast density from the two elements was separated using post-reconstruction spectral filtering to provide separate volume reconstruction for each agent: the gold channel mapped vascular permeability, while the iodine indicated the blood vessels.
Gold and iodine provide highest attenuation at different X-ray energies, and micro-CT imaging at 40 and 80 kVp gave the best differentiation. Data sets obtained using these two energies can be separated using a post-reconstruction decomposition method called bilateral filtration.
To determine whether their approach was feasible, Clark and co-workers first tested it using a 'digital phantom' to generate hypothetical data sets. After successfully resolving the two elements, the results were validated using a physical calibration phantom containing different concentrations of the two reagents used in the study. Minimum detectable concentrations were found to be 2.3 mg/mL (18mM) for iodine and 1.0 mg/mL (5.1 mM) for gold. Since much higher concentrations than these are readily achievable in vivo, this confirmed that the approach could be applied to real tumors.
Mouse tumor study shows successful dual imaging
with AuroVist™ and iodine in vivo
The investigators then took their method to the next level, imaging a soft-cell sarcoma in a mouse model. After administering 25 mg of AuroVist™-15, they imaged the animal on each of six successive days, monitoring the extravasation of the gold. On day 4, they administered the same quantity of liposomal iodine, imaging before and two hours after administration. The extended repeat imaging protocol allowed the identification of beam hardening artifacts, opening the possibility of removing a potential source of error in clinical applications.
Badea's lab have pioneered an important new technique in the fight against cancer. Vascular permeability and fractional blood volume are important markers for tumor state, and can be used to monitor the effects of both radiation and anti-angiogenic therapies on tumor growth and progression. Additionally, the ability to quantitate both these indicators simultaneously gives cancer researchers an important tool for testing new therapies, and the clarity of results has the potential for cutting the time needed to translate them into clinical use.
"We're really eager to see what these researchers will do next," said Dr. Hainfeld. "We created a new tool --AuroVist-- and they had a great idea for how to use it. This is science at its best, with many minds at work, moving us forward toward a cure."