![[Nanoprobes Tech Support (14k)]](../Images/logo_tech.gif)
Updated: December 7, 2006
PALMITOYL NANOGOLD®* PRODUCT INFORMATION
Palmitoyl Nanogold®
[Palmitoyl Nanogold® product page]

| Product Name: |
Palmitoyl Nanogold® |
| Catalog Number: |
4020 |
| Appearance: |
Dark brown solid |
| Revision: |
1.3 (December 2006) |
Technical Assistance Online
![[4020-PDF]](../Images/pdf.gif) Instructions (PDF) Instructions (PDF)
![[4020-PDF]](../Images/pdf.gif) Material Safety Data Sheet (PDF) Material Safety Data Sheet (PDF)
General Information
Palmitoyl-NANOGOLD® consists of the 1.4 nm NANOGOLD® particle covalently linked to a single palmitoyl molecule. Conjugation is via an amide linkage to the carboxylic group at the head of the molecule. It is intended as a lipid label for use with micelles and other dual-phase systems1 in a similar manner to the previously described undecatungstate membrane label.2 Its structure is shwon in figure 1:
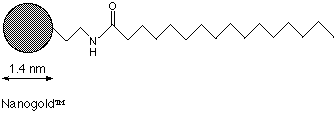
Figure 1: Structure of Palmitoyl NANOGOLD® (not shown to scale).
Palmitoyl NANOGOLD® is supplied as a solid, dried from methanol solution. It should be refrigerated upon receipt, and stored at -20°C.
* US Patent pending
Instructions for Use
Palmitoyl NANOGOLD® is hydrophobic, and can insert into organic phases in systems such as micelles.1 It is soluble in methanol and in methanol-trichloromethane and methanol-dichloromethane mixtures. Extinction coefficients at specific wavelengths are given below for methanol solution:
| WAVELENGTH (nm) |
EXTINCTION COEFFICIENT* |
| 280 |
2.25 X 105 |
| 420 |
1.12 X 105 |
* Measured for 5 X 10-6 M solution in methanol.
Heavily gold-labeled liposomes may be formed by dissolving the Palmitoyl-Nanogold® in chloroform, mixing with water, evaporating the chloroform, then using sonication or other usual preparative methods to form vesicles.1 Palmitoyl-Nanogold® may also be diluted with unlabeled lipids and used to prepare less heavily labeled liposomes. Incorporation of 0.1 to 1 % Palmitoyl-Nanogold® with an unlabeled lipid is usually appropriate for preparing Nanogold®-labeled liposomes with the same properties and morphology as those prepared without the gold label.Once incorporated into micelles or other structures, it may be used according to the procedures required by individual experiments in the same manner as unlabeled palmitic acid.
General Considerations for Staining with Nanogold® Reagents
Basically, normal methodologies may be used successfully with NANOGOLD® immunoreagents. The concentration of antibody and gold is similar to other commercial preparations of colloidal gold antibodies. Therefore similar dilutions and blocking agents are appropriate.
The major difference will be in the results:
- NANOGOLD® is an extremely uniform 1.4 nm diameter gold particle (ca.10%).
- NANOGOLD® conjugates contain absolutely no aggregates. This is in sharp contrast to other colloidal gold conjugates that usually are prepared by centrifugation to remove the largest aggregates and frequently contain smaller aggregates.
- Close to 1 NANOGOLD® particle to 1 probe molecule make this product distinct from the 0.2 - 10 variable stoichiometry of other colloidal gold antibody preparations.
- NANOGOLD® particles do not have affinity to proteins as do other other colloidal golds. This reduces background and false labeling.
- NANOGOLD® develops better with silver than do most other colloidal golds giving it higher sensitivity. Silver enhancement can be used to make the immunolabeling useful for light microscopy and immunoblotting with improved results.
Because the 1.4 nm NANOGOLD® particles are so small, over staining with OsO4, uranyl acetate or lead citrate may tend to obscure direct visualization of individual NANOGOLD® particles. Three recommendations for improved visibility of NANOGOLD® are:
- Use of reduced amounts or concentrations of usual stains.
- Use of lower atomic number stains such as NANOVAN, a Vanadium based stain.3
- Enhancement of NANOGOLD® with silver developers, such as LI SILVER or HQ SILVER.
Temperature Caution
Although NANOGOLD® is usually stable,4 under some conditions labeled specimens or conjugates may not be stable above 50°C. Best results are obtained at room temperature or 4°C. Avoid 37°C incubations. Use low temperature embedding media (e.g., Lowicryl) if labeling before embedding;5 do not bake tissue blocks with NANOGOLD®. If your experiment requires higher temperature embedding, then silver enhancement should be performed before embedding.
Thiol Caution
NANOGOLD® particles degrade upon exposure to concentrated thiols such as beta-mercaptoethanol or dithiothreitol. If such reagents must be used, concentrations should be kept below 1 mM and exposure restricted to 10 minutes or less.
Special Considerations for Direct Viewing of Nanogold® in the Electron Microscope
For most work, silver enhancement is recommended to give a good signal in the electron microscope (see below). For particular applications, visualization of the NANOGOLD® directly may be desirable. Generally this requires very thin samples and precludes the use of other stains.
NANOGOLD® provides a much improved resolution and smaller probe size over other colloidal gold antibody products. However, because NANOGOLD® is only 1.4 nm in diameter, it will not only be smaller, but will appear less intense than, for example, a 5 nm gold particle. With careful work, however, NANOGOLD® may be seen directly through the binoculars of a standard EM even in 80 nm thin sections. However, achieving the high resolution necessary for this work may require new demands on your equipment and technique. Several suggestions follow:
- Before you start a project with NANOGOLD® it is helpful to see it so you know what to look for. Dilute the NANOGOLD® stock 1:5 and apply 4 ml to a grid for 1 minute. Wick the drop and wash with deionized water 4 times.
- View NANOGOLD® at 100,000 X magnification with 10 X binoculars for a final magnification of 1,000,000 X. Turn the emission up full and adjust the condenser for maximum illumination.
- The alignment of the microscope should be in order to give 0.3 nm resolution. Although the scope should be well aligned, you may be able to skip this step if you do step 4
- Objective stigmators must be optimally set at 100,000 X. Even if the rest of the microscope optics are not perfectly aligned, adjustment of the objective stigmators may compensate and give the required resolution. You may want to follow your local protocol for this alignment but since it is important, a brief protocol is given here:
- At 100,000 X (1 X 106 with binoculars), over focus, under focus, then set the objective lens to in focus. This is where there is the least amount of detail seen.
- Adjust each objective stigmator to give the least amount of detail in the image.
- Repeat steps a and b until the in focus image contains virtually no contrast, no wormy details, and gives a flat featureless image.
- Now underfocus slightly, move to a fresh area, and you should see small black dots of 1.4 nm size. This is the NANOGOLD®. For the 1:5 dilution suggested, there should be about 5 to 10 gold spots on the small viewing screen used with the binoculars. Contrast and visibility of the gold clusters is best at 0.2 - 0.5 m defocus, and is much worse at typical defocus values of 1.5 - 2.0 m commonly used for protein molecular imaging.
- In order to operate at high magnification with high beam current, thin carbon film over fenestrated holey film is recommended. Alternatively, thin carbon or 0.2% Formvar over a 1000 mesh grid is acceptable. Many plastic supports are unstable under these conditions of high magnification/high beam current and carbon is therefore preferred. Contrast is best using thinner films and thinner sections.
- Once you have seen NANOGOLD® you may now be able to reduce the beam current and obtain better images on film. For direct viewing with the binoculars reduction in magnification from 1,000,000 X to 50,000 X makes the NANOGOLD® much more difficult to observe and not all of the golds are discernable. At 30,000 X (300,000 X with 10 X binoculars) NANOGOLD® particles are not visible. It is recommended to view at 1,000,000 X, with maximum beam current, align the objective stigmators, and then move to a fresh area, reduce the beam, and record on film.
- If the demands of high resolution are too taxing or your sample has an interfering stain, a very good result may be obtained using silver enhancement to give particles easily seen at lower magnification.
Silver Enhancement of Nanogold® for EM
NANOGOLD® will nucleate silver deposition resulting in a dense particle 2-80 nm in size or larger depending on development time. If specimens are to be embedded, silver enhancement is usually performed after embedding, although it may be done first. It must be completed before any staining reagents such as osmium tetroxide, lead citrate or uranyl acetate are applied, since these will nucleate silver deposition in the same manner as gold and produce non-specific staining. With NANOGOLD® reagents, low-temperature resins (eg Lowicryl) should be used and the specimens kept at or below room temperature until after silver development has been completed. Silver development is recommended for applications of NANOGOLD® in which these stains are to be used, otherwise the NANOGOLD® particles may be difficult to visualize against the stain.
Our LI SILVER silver enhancement system is convenient and not light sensitive, and suitable for all applications. Improved results in the EM may be obtained using HQ SILVER, which is formulated to give slower, more controllable particle growth and uniform particle size distribution.6
Specimens must be thoroughly rinsed with deionized water before silver enhancement reagents are applied. This is because the buffers used for antibody incubations and washes contain chloride ions and other anions which form insoluble precipitates with silver. These are often light-sensitive and will give non-specific staining. To prepare the developer, mix equal amounts of the enhancer and initiator immediately before use. NANOGOLD® will nucleate silver deposition resulting in a dense particle 2-20 nm in size or larger depending on development time. Use nickel grids (not copper).
The relevent procedure for immunolabeling should be followed up incubation with the NANOGOLD® reagent and wash. Silver enhancement is then performed as follows:
- Rinse with deionized water (2 X 5 mins).
- OPTIONAL (may reduce background): Rinse with 0.02 M sodium citrate buffer, pH 7.0 (3 X 5 mins).
- Float grid with specimen on freshly mixed developer for 1-8 minutes, or as directed in the instructions for the silver reagent. More or less time can be used to control particle size. A series of different development times should be tried, to find the optimum time for your experiment. With HQ silver, a development time of 6 min. gives 15-40 nm round particles.
- Rinse with deionized water (3 X 1 min).
- Mount and stain as usual.
Fixing with osmium tetroxide may cause some loss of silver; if this is found to be a problem, slightly longer development times may be appropriate.
NOTE: Treatment with osmium tetroxide followed by uranyl acetate staining can lead to much more drastic loss of the silver enhanced NANOGOLD® particles. This may be prevented by gold toning:7
- After silver enhancement, wash thoroughly with dionized water.
- 0.05 % gold chloride: 10 minutes at 4°C.
- Wash with deionized water.
- 0.5 % oxalic acid: 2 mins at room temperature.
- 1 % sodium thiosulfate (freshly made) for 1 hour.
- Wash thoroughly with deionized water and embed according to usual procedure.
Staining and Silver Enhancement with Nanogold® for Light Microscopy
Features labeled with NANOGOLD® will be stained black in the light microscope upon silver enhancement. Different development times should be tried to determine which is best for your experiment. The procedure for immunolabeling is similar to that for EM; a suitable procedure is given below.
Samples must be rinsed with deionized water before silver enhancement. This is because the reagent contains silver ions in solution, which react to form a precipitate with chloride, phosphate and other anions which are components of buffer solutions. The procedure for immunolabeling with NANOGOLD® and silver enhancement is given below.
- Rinse with deionized water (3 X 1 min).
- OPTIONAL (may reduce background): Rinse with 0.02 M sodium citrate buffer, pH 7.0 (3 X 5 mins).
- Develop specimen with freshly mixed developer for 5-20 minutes, or as directed in the instructions for the silver reagent. More or less time can be used to control intensity of signal. A series of different development times may be used, to find the optimum enhancement for your experiment; generally a shorter antibody incubation time will require a longer silver development time.
- Rinse with deionized water (2 X 5 mins).
- The specimen may now be stained if desired before examination, with usual reagents.
To obtain an especially dark silver signal, the silver enhancement may be repeated with a freshly mixed portion of developer.
Immunoblotting
The basic procedure for gold immunoblotting has been described by Moeremans et al.8 this may be used for experiments in which NANOGOLD® reagents are used in biological systems as part of blotting techniques. For best results, the membrane should be hydrated before use by simmering in gently boiling water for 15 minutes. Best results are obtained when the antigen is applied using a 1 microliter capillary tube. The procedure for immunoblots is as follows:
- Spot 1 microliter dilutions of the target in buffer 4 onto hydrated nitrocellulose membrane. Use an antigen concentration range from 100 ng to 0.01 pg / microliter.
- Block with buffer 1 for 30 minutes at 37°C.
- Incubate with a solution of the DPPE-NANOGOLD® product (such as a micelle) in buffer 2 for 2 hours at room temperature.
- Rinse with buffer 3 (3 X 5 mins), then buffer 4 (2 X 5 mins).
- OPTIONAL (may improve sensitivity): Postfix with glutaraldehyde, 1 % in buffer 4 (10 mins).
- Rinse with deionized water (2 X 5 mins).
- OPTIONAL (may reduce background): Rinse with 0.05 M EDTA at pH 4.5 (5 mins).
- Develop with freshly mixed silver developer for 20-25 minutes or as directed in the instructions for the silver reagent, twice. Rinse thoroughly with deionized water between developments to remove all the reagent.
- Rinse several times with deionized water.
CAUTION: NANOGOLD® particles degrade upon exposure to concentrated thiols such as beta-mercaptoethanol or dithiothreitol. If such reagents must be used, concentrations should be kept below 1 mM and exposure restricted to 10 minutes or less.
| Buffer 1:
20 mM phosphate
150 mM NaCl
pH 7.40
4% BSA (bovine serum albumin)
2 mM sodium azide (NaN3) |
Buffer 2:
20 mM phosphate
150 mM NaCl
pH 7.4
0.8% BSA
1% normal serum; use serum of the host animal for the NANOGOLD® antibody
0.1% gelatin (Type B, approx. 60 bloom)
Optional, may reduce background:
0.5 M NaCl
0.05% Tween 20
|
| Buffer 3:
20 mM phosphate
150 mM NaCl
pH 7.40
0.8% BSA (bovine serum albumin)
2 mM sodium azide |
Buffer 4 (PBS):
20 mM phosphate
150 mM NaCl
pH 7.4 |
Other procedures may be used; for example the NANOGOLD® reagent may be used as a tertiary labeled probe. If antibody incubation steps are used, rinse with buffer 3 (3 X 10 mins) after incubation.
- Hainfeld, J. F.: Gold Liposomes. In Proc 54th Ann. Mtg. Micros. Soc. Amer., G. W. Bailey, J. M. Corbett, R. V. W. Dimlich, J. R. Michael and N. J., Zaluzec (Eds.). San Francisco Press, San Francisco, CA, pp. 898-899 (1996).
- Hainfeld, J. F.; Lipka, J. J., and Quaite, F. E.; J. Histochem. Cytochem., 38, 1795 (1990).
- Tracz, E., Dickson, D. W., Hainfeld, J. F., and Ksiezak-Reding, H. Brain Res., 773, 33-44 (1997); Gregori, L., Hainfeld, J. F., Simon, M. N., and Goldgaber, D. Binding of amyloid beta protein to the 20S proteasome. J. Biol. Chem., 272, 58-62 (1997); Hainfeld, J. F.; Safer, D.; Wall, J. S.; Simon, M. N.; Lin, B. J., and Powell, R. D.; Proc. 52nd Ann. Mtg., Micros. Soc. Amer.; G. W. Bailey and Garratt-Reed, A. J., (Eds.); San Francisco Press, San Francisco, CA, 1994, p. 132.
- Hainfeld, J. F., and Furuya, F. R.; in Immunogold-Silver Staining: Principles, Methods and Applications (M. A. Hayat, Ed.), CRC Press, Boca raton, FL., 1995: pp. 71-96.
- Krenács, T., and Krenács, L.; in Immunogold-Silver Staining: Principles, Methods and Applications (M. A. Hayat, Ed.), CRC Press, Boca raton, FL., 1995: pp. 57-69.
- Humbel, B. M.; Sibon, O. C. M.; Stierhof, Y.-D., and Schwarz, H.: J. Histochem. Cytochem., 43, 735-737 (1995).
- Arai, R., et al.; Brain Res. Bull. 28, 343-345 (1992).
- Moeremans, M. et al.; J. Immunol. Meth., 74, 353 (1984).
Technical Assistance Available.
For a complete list of references citing this product, please visit our References page.
|