![[Nanoprobes Tech Support (14k)]](../Images/logo_tech.gif)
Updated: March 26, 2000
UNDECAGOLD PRODUCT INFORMATION
Mono-Sulfo-NHS-Undecagold Labeling Reagent
[Mono-Sulfo-NHS-Undecagold Labeling Reagent product page]

| Product Name: |
SULFOSUCCINIMIDO UNDECAGOLD, 5 X 10 nmol |
| Catalog Number: |
2045A |
| Appearance: |
Orange-yellow powder or solid |
| Revision: |
1.4 (March 2000) |
Technical Assistance Online
![[2045A-PDF]](../Images/pdf.gif) Instructions (PDF) Instructions (PDF)
![[2045A-PDF]](../Images/pdf.gif) Material Safety Data Sheet (PDF) Material Safety Data Sheet (PDF)
Congratulations on your acquisition of a revolutionary new gold immunoreagent: the sulfo-SUCCINIMIDO UNDECAGOLD labeling kit. With this reagent you can label your own proteins, or any other biomolecule containing an accessible sulfhydryl group, with UNDECAGOLD. Because UNDECAGOLD is a discrete molecular compound and not a colloidal gold preparation, conjugates prepared with this reagent have several advantages over colloidal gold conjugates (see below). It is the smallest gold probe commercially available, with a diameter of just 0.8 nm.
Guide to Gold Cluster Labeling Our detailed description of gold cluster labeling, how to isolate conjugates and calculate the extent of labeling, is now available. This new section of our site includes spectra, micrographs, and tips and suggestions for better labeling (opens in a new window).
Warning: For research use only. Not recommended or intended for diagnosis of disease in humans or animals. Do not use internally or externally in humans or animals. Non radioactive and non carcinogenic.
UNDECAGOLD is the smallest gold label available, prepared using a discrete gold compound rather than a colloid.1 This kit contains the UNDECAGOLD particle with a reactive sulfo-succinimide functionality incorporated into a ligand on the surface of the gold particle; this has a specific reactivity towards primary amines, and may be covalently linked to any protein bearing an accessible primary amine, as shown on the next page (figure 1). The reagent as supplied has been lyophilized from 0.1 M HEPES-HCl at pH 4.0; dissolution in 0.2 ml deionized water will produce a solution of activated UNDECAGOLD in this buffer. UNDECAGOLD conjugates are intended for use in high resolution electron microscopy where the smallest possible probe and the lowest possible interference with the immunoreactivity of the conjugate are desired. They are stable to wide ranges of pH and ionic strength, and are not radioactive or carcinogenic. The labeling reagent should be stored at -20°C.
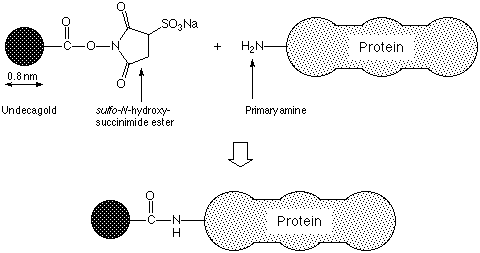
Figure 1: Schematic showing UNDECAGOLD labeling of a protein via reaction of an amine and a sulfo-succinimide group.
This product contains 10 nmol of activated undecagold reagent: this is sufficient to label up to 2 nmol of amine sites. This corresponds to 0.2 mg of a protein with a molecular weight of 100,000 and a single labeling site.
Contents
UNDECAGOLD particles degrade upon exposure to concentrated thiols such as beta-mercaptoethanol or dithiothreitol. If such reagents must be used, concentrations should be kept below 1 mM and exposure restricted to 10 minutes or less.
Contents
For most proteins no pretreatment is necessary since amines will be present. The protein is reacted with the undecagold2 in buffer solution at pH 7.5, either for 1 hour at room temperature or overnight at 4°C; the pH shopuld be adjusted to 7.5 after mixing. The UNDECAGOLD conjugated product may be isolated either by gel filtration, using a fine gel such as Pharmacia Superose 6 or 12, Superdex 75 or Amicon GCL-90. Alternatively, unbound UNDECAGOLD may be removed by dialysis. The recommended procedure is given below:
- Dissolve the protein in 0.02 M sodium phosphate buffer at pH 7.4 with 150 mM sodium chloride (1 mL)
- Dissolve the UNDECAGOLD reagent in 0.2 ml deionized water. Sufficient reagent is supplied to label 10 nmol of amine sites; if you are using a smaller amount, use a proportionately smaller amount of the UNDECAGOLD reagent (e.g. half the reagent supplied will label 0.15 mg of IgG). Once activated UNDECAGOLD is reconstituted with water it should be used immediately. The succinimide ester is hydrolyzed in aqueous solution.
- Add the activated UNDECAGOLD solution to the reduced antibody, and raise the pH of the mixture carefully to 7.5 with dilute sodium hydroxide. Incubate for either 1 hour at room temperature, or 12-18 hours at 4°C.
- Separate the unbound gold particles from the antibody conjugates using dialysis or gel exclusion chromatography.
(a) DIALYSIS: Use a membrane with a molecular weight cutoff of 100,000 and as the exchange buffer use 0.02 M sodium phosphate at pH 7.4 with 150 mM sodium chloride; stir for 4-6 hours in 200 ml of buffer. Repeat twice with fresh buffer. The membrane will retain the UNDECAGOLD labeled antibody, but unbound UNDECAGOLD particles and any other low molecular weight impurities will pass through.
(b) GEL FILTRATION: The UNDECAGOLD conjugate may be effectively isolated by HPLC using a gel such as Pharmacia Superose 6 or 12 (which fractionate a wide range of molecular weights) or Amicon GCL-90 (which excludes molecules of mass 30,000 or greater). Concentrate the reaction mixture to a suitably small volume using membrane centrifugation (e.g. Amicon Centricon-30 system). Elute with 0.02 M sodium phosphate at pH 7.4 with 150 mM sodium chloride; the first, pale yellow peak or shoulder is the conjugate, while the second, darker band is unbound UNDECAGOLD particles. For even higher purity, repeat the this process one more time.
The extent of labeling may be calculated from the UV/visible spectrum of the conjugate. UNDECAGOLD has the following extinction coefficients at specific wavelengths:
| WAVELENGTH (nm) |
EXTINCTION COEFFICIENT* |
| 280 |
1.7 X 105 |
| 420 |
0.47 X 105 |
* Measured for 5 X 10-6 M solution in methanol.
UNDECAGOLD conjugates should be stored in 0.02 M sodium phosphate buffer with 150 mM sodium chloride; if they are to be stored longer than three days, add 0.1 % bovine serum albumin and 0.05 % sodium azide to prevent bacterial contamination and to prevent the protein from adhering to the surfaces of the storage vessel.
Contents
The procedure described below is suggested for labeling smaller peptides (MW 2,000 or less); these are significantly smaller than the UNDECAGOLD particle (molecular weight approximately 5,000). In this case separation of excess, unreacted peptide from gold-peptide conjugates is usually easier than separating conjugates from unbound gold, and therefore it is adviseable to use an excess of the peptide to be labeled. The protein is reacted with the Nanogold in buffer solution at pH 7.5-8, either for 1 hour at room temperature or overnight at 4°C; the pH should be near 8.0 after mixing. The UNDECAGOLD conjugated product may be isolated by gel filtration, using gel which can separate compounds of MW 5,000 and below, such as Pharmacia Superdex-Peptide or Superdex-75, Bio-Rad Bio-Gel P2 or P6, or a desalting gel such as Amicon GH25 (exclusion limit 3,000 MW). Alternatively, dialysis may be used.
- Dissolve the peptide in 0.02 M HEPES buffer, adjusted to pH 7.5 with dilute sodium hydroxide (0.2 mL).
- Dissolve the mono-sulfo-NHS-UNDECAGOLD reagent in 0.2 ml deionized water. 50 nmol of reagent is supplied; use a 5 to 20-fold exess of the peptide to be labeled. Once activated UNDECAGOLD is reconstituted with water it should be used immediately. The succinimide ester is hydrolyzed in aqueous solution).
- Add the activated UNDECAGOLD solution to the dissolved protein. Incubate for either 1 hour at room temperature, or 12-18 hours at 4°C.
- Separate the gold-peptide conjugates from excess, unbound peptide using gel exclusion chromatography or dialysis
(a) DIALYSIS: Use a membrane with a molecular weight cutoff between the MW of the peptide and 5,000, and as the exchange buffer use 0.02 M sodium phosphate at pH 7.4 with 150 mM sodium chloride; stir for 4-6 hours in 200 ml of buffer. Repeat twice with fresh buffer. The membrane will retain the UNDECAGOLD labeled peptide, but unbound peptide and any other low molecular weight impurities will pass through.
(b) GEL FILTRATION: The UNDECAGOLD conjugate may be effectively isolated by HPLC using a gel such as Pharmacia Superdex-Peptide or Superdex-75, or bio-Rad Bio-Gel P2 or P6 (MW of UNDECAGOLD is near 5,000). Concentrate the reaction mixture to a suitably small volume using membrane centrifugation (e.g. Amicon Centricon-3 system). Elute with 0.02 M sodium phosphate at pH 7.4 with 150 mM sodium chloride; the first, pale yellow-brown peak or shoulder is the conjugate, while the second, colorless band is excess peptide. For even higher purity, repeat the this process one more time.
If the extinction coefficient of the peptide is known, the labeling may be calculated; Sulfo-succinimido-UNDECAGOLD has extinction coefficients at 280 nm of 1.7 X 105, and at 420 nm of 0.47 X 105. However, smaller peptides often have much lower extinction coefficients which may lead to inaccuracies in calculations.
UNDECAGOLD conjugates should be stored in 0.02 M sodium phosphate buffer with 150 mM sodium chloride; if they are to be stored longer than three days, add 0.1 % bovine serum albumin and 0.05 % sodium azide to prevent bacterial contamination and to prevent the protein from adhering to the surfaces of the storage vessel.
NOTE: Proteins with MW close to that of the UNDECAGOLD particle (5,000) may be labeled using SULFO-N-HYDROXY-SUCCIN-IMIDO UNDECAGOLD, but the products may be less effectively separated by gel filtration. Other chromatographic methods, such as reverse-phase, hydrophobic interaction or ion exchange chromatography, may be used. UNDECAGOLD is more hydrophobic than most proteins and elutes differently. AN UNDECAGOLD-peptide conjugate has intermediate characteristics.
Contents
Basically, normal methodologies may be used successfully with UNDECAGOLD immunoreagents. The concentration of antibody and gold is similar to other commercial preparations of colloidal gold antibodies. Therefore similar dilutions and blocking agents are appropriate.
The major difference will be in the results:
- UNDECAGOLD is an extremely uniform 0.8 nm diameter gold particle (ca.10%).
- UNDECAGOLD conjugates contain absolutely no aggregates. This is in sharp contrast to other colloidal gold conjugates that usually are prepared by centrifugation to remove the largest aggregates and frequently contain smaller aggregates.
- Close to 1 UNDECAGOLD particle to 1 Fab' (or IgG) make this product distinct from the 0.2 - 10 variable stoichiometry of other colloidal gold antibody preparations.
- UNDECAGOLD particles do not have affinity to proteins as do other other colloidal golds. This reduces background and false labeling.
Contents
Because the 0.8 nm UNDECAGOLD particles are so small, over staining with osmium tetroxide, uranyl acetate or lead citrate will obscure direct visualization of individual UNDECAGOLD particles, and therefore these stains should not ber used. Only light staining with a low atomic number stain, such as NANOVAN, a Vanadium based negative stain, should be used.
Contents
If aldehyde-containing reagents have been used for fixation, these must be quenched before labeling. This may be achieved by incubating the specimens for 5 minutes in 50 mM glycine solution in PBS (pH 7.4). Ammonium chloride (50 mM) or sodium borohydride (0.5 - 1 mg/ml) in PBS may be used instead of glycine.
Cells in Suspension
- Optional fixing of cells: e.g., with glutaraldehyde (0.05 - 1% for 15 minutes) in PBS. Do not use Tris buffer since this contains an amine. After fixation, centrifuge cells (e.g. 1 ml at 10,000,000 cells/ml) at 300 X g, 5 minutes; discard supernatant; resuspend in 1 ml buffer. Repeat this washing (centrifugation and resuspension) 2 times.
- Incubate cells with 0.02 M glycine in PBS (5 mins). Centrifuge, then resuspend cells in PBS-BSA buffer (specified below) for 5 minutes.
- Wash cells using PBS-BSA as described in step 1 (2 X 5 mins). Resuspend in 1 ml Buffer 1.
- Place 50 - 200 microliters of cells into Eppendorf tube. Dilute UNDECAGOLD conjugate ~ 50 times in PBS-BSA buffer and add 30 microliters to cells; incubate for 30 minutes with occasional shaking (do not create bubbles which will denature proteins).
- Wash cells in PBS-BSA as described in step 1 (2 X 5 mins).
- Fix cells and antibodies using a final concentration of 1% glutaraldehyde in PBS for 15 minutes. Then remove fixative by washing with buffer 1 (3 X 5 mins).
| PBS-BSA Buffer:
20 mM phosphate
150 mM NaCl
pH 7.40
0.5% BSA
0.1% gelatin (high purity)
Optional, may reduce background:
0.5 M NaCl
0.05% Tween 20 |
PBS Buffer:
20 mM phosphate
150 mM NaCl
pH 7.40 |
Negative staining may be used for electron microscopy of small structures or single molecules which are not embedded. Negative stain must be applied after the silver enhancement. NANOVAN negative stain is specially formulated for use with UNDECAGOLD reagents; it is based on vanadium, which gives a lighter stain than uranium, lead or tungsten-based negative stains and allows easier visualization of UNDECAGOLD particles with little or no silver enhancement.
Thin Sections
Labeling with UNDECAGOLD may be performed before or after embedding.3 Labeling before embedding and sectioning (the pre-embedding method) is used for the study of surface antigens, particularly small organisms such as viruses budding from host cells. It gives good preservation of cellular structure, and subsequent staining usually produces high contrast for study of the cellular details. Labeling after embedding and sectioning (the post-embedding method) allows the antibody access to the interior of the cells, and is used to label both exterior and interior features. The procedures for both methods are described below.
Thin sections mounted on grids are floated on drops of solutions on parafilm or in well plates. Hydrophobic resins usually require pre-etching.
PROCEDURE FOR PRE-EMBEDDING METHOD:3
- Float on a drop of water for 5 - 10 minutes.
- Incubate cells with 1 % bovine serum albumin in PBS buffer at pH 7.4 for 5 minutes; this blocks any non-specific protein binding sites and minimizes non-specific antibody binding.
- Rinse with PBS-BSA (1 min).
- Incubate with UNDECAGOLD conjugate diluted 1/40 - 1/200 in PBS-BSA with 1 % normal serum from the same species as the UNDECAGOLD labeled antibody, for 10 minutes to 1 hour at room temperature.
- Rinse with PBS-BSA (3 X 1 min), then PBS (3 X 1 min).
- Postfix with 1 % glutaraldehyde in PBS (10 mins).
- Rinse in deionized water (2 X 5 min).
- Dehydrate and embed according to usual procedure. Use of a low-temperature resin (eg. Lowicryl) is recommended.
- Stain (uranyl acetate, lead citrate or other positive staining reagent) as usual before examination.
Silver enhancement may be performed before or after embedding (see below); it should be completed before postfixing or staining with osmium tetroxide, uranyl acetate or similar reagents is carried out.
PROCEDURE FOR POST-EMBEDDING METHOD:3
- Prepare sections on plastic or carbon-coated nickel grid. Float on a drop of water for 5 - 10 minutes.
- Incubate with 1 % solution of bovine serum albumin in PBS buffer at pH 7.4 for 5 minutes to block non-specific protein binding sites.
- Rinse with PBS-BSA (1 min).
- Incubate with UNDECAGOLD conjugate diluted 1/40 - 1/200 in PBS-BSA with 1 % normal serum from the same species as the UNDECAGOLD labeled antibody, for 10 minutes to 1 hour at room temperature.
- Rinse with PBS (3 X 1 min).
- Postfix with 1 % glutaraldehyde in PBS at room temperature (3 mins).
- Rinse in deionized water for (2 X 5 min).
- If desired, contrast sections with uranyl acetate and/or lead citrate before examination.
Silver enhancement may also be used to render the UNDECAGOLD particles more easily visible (see below), especially if stains such as uranyl acetate or lead citrate are applied. If used, it should be completed before these stains are applied.
| PBS-BSA Buffer:
20 mM phosphate
150 mM NaCl
pH 7.40
0.5% BSA
0.1% gelatin (high purity)
Optional, may reduce background:
0.5 M NaCl
0.05% Tween 20 |
PBS Buffer:
20 mM phosphate
150 mM NaCl
pH 7.40 |
Contents
UNDECAGOLD is the smallest gold probe commercially available, being just 0.8 nm in diameter. A high resolution instrument such as a Scanning Transmission Eelectron Microscope (STEM) is required for visualization; in a conventional TEM the UNDECAGOLD particles are not visible. With careful work, however, UNDECAGOLD may be seen directly in the STEM. However, achieving the high resolution necessary for this work may require new demands on your equipment and technique. Several suggestions follow:
- Before you start a project with UNDECAGOLD it is helpful to see it so you know what to look for. Dilute the UNDECAGOLD stock 1:5 in methanol and apply 4 microliters to a grid for 1 minute. Allow to dry.
- View UNDECAGOLD using a full width scan of 128 nm or less; this will give sufficient magnification for visualization.
- UNDECAGOLD is sensitive to beam damage (contrary to NANOGOLD® which is very beam-resistant); the behavior of UNDERCAGOLD in the STEM has been described in the literature4 Image at approximately 200 eÅ-2.
- In order to operate at high magnification, thin carbon film over fenestrated holey film is recommended. Many plastic supports are unstable under these conditions of high magnification/high beam current and carbon is therefore preferred. Contrast is best using thinner films.
Contents
UNDECAGOLD will nucleate silver deposition resulting in a dense particle 2-20 nm in size or larger depending on development time. However, silver enhancement will be slower and much less uniform than with larger gold particles such as NANOGOLD®. If specimens are to be embedded, silver enhancement is usually performed after embedding, although it may be done first. It must be completed before any staining reagents such as osmium tetroxide, lead citrate or uranyl acetate are applied, since these will nucleate silver deposition in the same manner as gold and produce non-specific staining.
Our LI SILVER silver enhancement system is convenient and not light sensitive, and suitable for all applications. Improved results in the EM may be obtained using HQ SILVER, which is formulated to give slower, more controllable particle growth and uniform particle size distribution.
Specimens must be thoroughly rinsed with deionized water before silver enhancement reagents are applied. This is because the buffers used for antibody incubations and washes contain chloride ions and other anions which form insoluble precipitates with silver. These are often light-sensitive and will give non-specific staining. To prepare the developer, mix equal amounts of the enhancer and initiator immediately before use. UNDECAGOLD will nucleate silver deposition resulting in a dense particle 2-20 nm in size or larger depending on development time. Use nickel grids (not copper).
The procedure for immunolabeling should be followed up to step 6 as described above. Silver enhancement is then performed as follows:
- Rinse with deionized water (2 X 5 mins).
- Float grid with specimen on freshly mixed developer for 1-4 minutes, or as directed in the instructions for the silver reagent. More or less time can be used to control particle size. A series of different development times should be tried, to find the optimum time for your experiment.
- Rinse with deionized water (3 X 1 min).
- Mount as usual.
Contents
For most light microscopy applications we recommend NANOGOLD® conjugates, since these usually give more intense, specific signals. UNDECAGOLD must be developed with a silver enhancement reagent before it is visible in the light microscope. Our LI SILVER is convenient for this as it is not light sensitive and the degree of enhancement may be monitored readily.
Samples must be rinsed with deionized water before silver enhancement. This is because the reagent contains silver ions in solution, which react to form a precipitate with chloride, phosphate and other anions which are components of buffer solutions. The procedure for immunolabeling with UNDECAGOLD and silver enhancement is given below.
- Spin cells onto slides using Cytospin, or use parafin section.
- Incubate with 1 % solution of bovine serum albumin in PBS (PBS-BSA) for 10 minutes to block non-specific protein binding sites.
- Incubate with primary antibody, diluted at usual working concentration in PBS-BSA (1 hour or usual time)
- Rinse with PBS-BSA (3 X 2 min).
- Incubate with UNDECAGOLD reagent diluted 1/40 - 1/200 in PBS-BSA with 1 % normal serum from the same species as the UNDECAGOLD reagent, for 1 hour at room temperature.
- Rinse with PBS (3 X 5 min).
- Postfix with 1 % glutaraldehyde in PBS at room temperature (3 mins).
- Rinse with deionized water (3 X 1 min).
- Develop specimen with freshly mixed developer for 5-20 minutes, or as directed in the instructions for the silver reagent. More or less time can be used to control intensity of signal. A series of different development times may be used, to find the optimum enhancement for your experiment; generally a shorter antibody incubation time will require a longer silver development time.
- Rinse with deionized water (2 X 5 mins).
- The specimen may now be stained if desired before examination, with usual reagents.
| PBS-BSA Buffer:
20 mM phosphate
150 mM NaCl
pH 7.40
0.5% BSA
0.1% gelatin (high purity)
Optional, may reduce background:
0.5 M NaCl
0.05% Tween 20 |
PBS Buffer:
20 mM phosphate
150 mM NaCl
pH 7.40 |
To obtain an especially dark silver signal, the silver enhancement may be repeated with a freshly mixed portion of developer.
Contents
For immunoblotting we recommend our NANOGOLD® conjugates, since these give more sensitive detection than UNDECAGOLD conjugates with a higher signal-to-noise ratio upon silver enhancement. The basic procedure for gold immunoblotting has been described by Moeremans et al,5 which may be followed. For best results, the membrane should be hydrated before use by simmering in gently boiling water for 15 minutes. Best results are obtained when the antigen is applied using a 1 microliters capillary tube. A suitable procedure for immunoblots is as follows:
- Spot 1 microliters dilutions of the antigen in buffer 4 onto hydrated nitrocellulose membrane. Use an antigen concentration range from 100 ng to 0.01 pg / microliter.
- Block with buffer 1 for 30 minutes at 37°C.
- Incubate with primary antibody according to usual procedure (usually 1 or 2 hours).
- Rinse with buffer 1 (3 X 10 mins).
- Incubate with a 1/100 to 1/200 dilution of the UNDECAGOLD reagent in buffer 2 for 2 hours at room temperature.
- Rinse with buffer 3 (3 X 5 mins), then buffer 4 (2 X 5 mins).
- OPTIONAL (may improve sensitivity): Postfix with glutaraldehyde, 1 % in buffer 4 (10 mins).
- Rinse with deionized water (2 X 5 mins).
- OPTIONAL (may reduce background): Rinse with 0.05 M EDTA at pH 4.5 (5 mins).
- Develop with freshly mixed silver developer for 20-25 minutes or as directed in the instructions for the silver reagent, twice. Rinse thoroughly with deionized water between developments to remove all the reagent.
- Rinse several times with deionized water.
CAUTION: UNDECAGOLD particles degrade upon exposure to concentrated thiols such as beta-mercaptoethanol or dithiothreitol. If such reagents must be used, concentrations should be kept below 1 mM and exposure restricted to 10 minutes or less.
| Buffer 1:
20 mM phosphate
150 mM NaCl
pH 7.40
4% BSA (bovine serum albumin)
2 mM sodium azide (NaN3) |
Buffer 2:
20 mM phosphate
150 mM NaCl
pH 7.4
0.8% BSA
1% normal serum; use serum of the host animal for the NANOGOLD® antibody
0.1% gelatin (Type B, approx. 60 bloom)
Optional, may reduce background:
0.5 M NaCl
0.05% Tween 20
|
| Buffer 3:
20 mM phosphate
150 mM NaCl
pH 7.40
0.8% BSA (bovine serum albumin)
2 mM sodium azide |
Buffer 4 (PBS):
20 mM phosphate
150 mM NaCl
pH 7.4 |
Other procedures may be used; for example the UNDECAGOLD reagent may be used as a tertiary labeled antibody, or a custom UNDECAGOLD conjugate may be the primary antibody. If additional antibody incubation steps are used, rinse with buffer 3 (3 X 10 mins) after incubation.
Contents
- Hainfeld, J. F.; in "Colloidal Gold: Principles, Methods and Applications;" M. A. Hayat, ed.; Vol. 1, p. 413; Academic Press, San Diego, CA (1991).
- Lee, W. T., and Conrad, D. H.; J. Exp. Med., 159, 1790 (1984).
- J. E. Beesley, in "Colloidal Gold: Principles, Methods and Applications," M. A. Hayat, ed., Academic Press, New York, 1989; Vol. 1, pp. 421-425.
- Lipka, J. J., Hainfeld, J. F., and Wall, J. S., J. Ultrastruct. Res., 84, 120 (1983).
- Moeremans, M. et al., J. Immunol. Meth., 74, 353 (1984).
Contents
|