![[Nanoprobes Tech Support (14k)]](../Images/common_images/2013-11-15-Nanoprobes-Color_Letterhead-w-tech.jpg)
GOLDIBLOT PRODUCT INFORMATION
GoldiBlot™ His-Tag Western Blot Kit
[GoldiBlot His-Tag Western Blot Kit product page]

| Product Name: |
GoldiBlot™ His-Tag Western Blot Kit |
| Catalog Number: |
2090, 2090A |
| Revision: |
1.2 (June 2014) |
Technical Assistance Online
![[2080-PDF]](../Images/pdf.gif) Instructions (PDF) Instructions (PDF)
Warning: For research use only. Not recommended or intended for diagnosis of disease in humans or animals. Do not use internally or externally in humans or animals. Non radioactive and non carcinogenic.
GoldiBlot™ His-Tag Western Blot Kit is intended and optimized for direct visualization of recombinant His-tagged proteins, and other proteins bearing histidine tags in western or dot blotting applications.
GoldiBlot™ improves greatly upon standard detection by anti-6xHis antibodies:
- Does not require a specific location of the polyhistidine tag (N- or C-terminus)
- No need for specific adjacent amino acid sequences
- No incubation time --No primary or secondary antibodies needed!
GoldiBlot™ His-Tag Western Blot Kit uses Ni-NTA (nickel-nitrilotriacetic acid)-functionalized gold nanoparticles to specifically and directly bind to His-tagged proteins1-6. With autometallographic amplification subsequently applied to the gold nanoparticles, GoldiBlot™ allows the direct visualization of His-tagged proteins. GoldiBlot™ generates specific, purple-colored metallic bands or dots, which do not fade and will not dissolve in water and organic solvents. The GoldiBlot™ His-Tag Western Blot Kit can detect nanogram levels of purified His-tagged proteins, and detects His-tagged proteins in crude extract as well. The entire procedure takes about 1 hour.
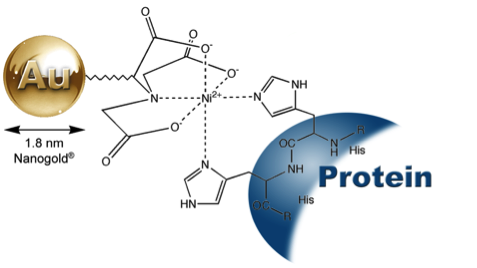
GoldiBlot™ (Ni-NTA-Nanogold®), showing mechanism of binding
to a polyhistidine (His) – tagged protein. |
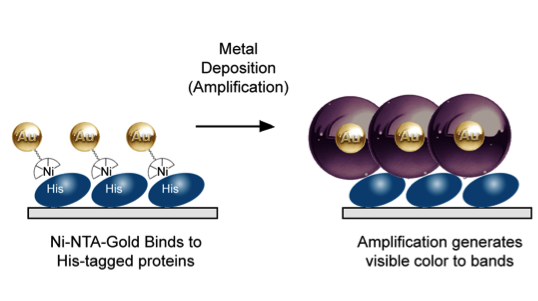
Principle of GoldiBlot™: gold binding followed by autometallographic amplification (deposition of metal selectively onto the gold particles) generates visible signal. |
The following materials are sufficient for 15 mini-blots (7 cm x 8.4 cm) of membrane (kit #2090):
| GoldiBlot™ Nickel-NTA-Nanogold® |
1.5 mL |
| GoldiBlot™ AutoMet Detect A |
40 mL |
| GoldiBlot™ AutoMet Detect B |
40 mL |
| GoldiBlot™ AutoMet Detect C |
40 mL |
| GoldiBlot™ AutoMet Detect D |
40 mL |
- TBS-0.1%T: 20 mM Tris, 0.15 M NaCl, pH7.6, 0.1% (w/v) Tween® -20
- 5 % (w/v) nonfat dry milk in TBS-0.1%T
- TBS-0.6%T: 20 mM Tris, 0.15 M NaCl, pH7.6, 0.6% (w/v) Tween® -20
- 1 % (w/v) nonfat dry milk in TBS-0.6%T
- 10 mM imidazole in TBS-0.6%T
Refrigerate at 4°C. The product is shipped at ambient temperature.
Note: Volumes indicated below are for one 7 cm x 8.4 cm blot. Volumes can be adjusted for staining multiple blots or for one different-sized blot.
All GoldiBlot™ reagents and other required materials should be equilibrated to room temperature prior to the western blotting procedure. All incubations of the GoldiBlot™ western blotting are performed at room temperature with shaking.
- Transfer proteins from gel to a PVDF membrane.
Note: Although other membranes can be used, optimization may be required.
- Place the membrane in a tray and equilibrate with TBS-0.1%T for 3 min.
- Block the membrane with 5 % (w/v) nonfat dry milk in TBS-0.1%T for 15 min.
- Add 0.1 ml of GoldiBlot™ Nickel-NTA-Nanogold® to 10 ml of 1 % (w/v) nonfat dry milk in TBS-0.6%T. Vortex. Place the membrane in the solution, and incubate the blot for 30 min.
- Wash the membrane two times with 15 ml of 10 mM imidazole in TBS-0.6%T for 2 min each.
- Wash the membrane three times with 15 ml of deionized water for 3 min each.
- Before starting the last deionized water wash, mix 2.5 ml GoldiBlot™ AutoMet Detect A with 2.5 ml B in a clean 15 ml container. After 5 min, add 2.5 ml C and 2.5 ml D to the mixture of A and B, and mix. Incubate the blot with 10 ml of the ABCD mixture for 6 to 15 min, or until satisfactory staining is reached.
Note: The incubation time of GoldiBlot™ AutoMet Detect ABCD depends on the quantities of His-tagged proteins loaded. The bands loaded with more than 100 ng His-tagged proteins can be seen within 6 min. Longer incubation time may be needed in order to see less than 20 ng His-tagged proteins. However, longer incubation may lead to the visualization of some non specific background bindings.
- Wash the membrane three times with 15 ml of deionized water for 3 min each to terminate the autometallographic amplification.
Note: The light purple-colored membrane background fades away as the membrane dries out.
- Air-dry the membrane.
Note: The concentration of NaCl and Tween 20 in binding and washes (used in GoldiBlot™ Nickel-NTA-Nanogold® binding and imidazole washes) can be slightly adjusted to achieve an optimized signal-to-noise ratio. Less NaCl and Tween20 can enhance the band intensity of His-tagged proteins, and higher NaCl and Tween 20 help reduce the nonspecific background staining.
- Hochuli, E.; Dobeli H, Schacher A. New metal chelate adsorbent selective for proteins and peptides containing neighbouring histidine residues. J. Chromatograph., 411, 177-184 (1987).
- Schmitt, J.; Hess, H., and Stunnenberg, H. G.: Affinity purification of histidine-tagged proteins. Molecular Biology Reports, 18, 223-230 (1993).
- Hainfeld, J. F.; Liu, W.; Halsey, C. M. R.; Freimuth, P., and Powell, R. D.: Ni-NTA-Gold clusters target His-tagged proteins. J. Struct. Biol., 127, 185-198 (1999).
- Collins, R. F.; Beis, K.; Clarke, B. R.; Ford, R. C.; Hulley, M.; Naismith, J. H.; and Whitfield, C.: Periplasmic protein-protein contacts in the inner membrane protein Wzc form a tetrameric complex required for the assembly of Escherichia coli group 1 capsules. J. Biol. Chem., 281, 2144-2150 (2006).
- Wolfe, C. L.; Warrington, J. A.; Treadwell, L., and Norcum, M. T.: A three-dimensional working model of the multienzyme complex of aminoacyl-tRNA synthetases based on electron microscopic placements of tRNA and proteins. J. Biol. Chem., 280, 38870-38878 (2005).
- Bumba, L.; Tichy, M.; Dobakova, M.; Komenda, J., and Vacha, F.: Localization of the PsbH subunit in photosystem II from the Synechocystis 6803 using the His-tagged NiNTA Nanogold labeling. J. Struct. Biol., 152, 28-35 (2005).
Need help?
|